A manual of elementary chemistry : theoretical and practical / by George Fownes.
- Fownes, George, 1815-1849.
- Date:
- 1868
Licence: Public Domain Mark
Credit: A manual of elementary chemistry : theoretical and practical / by George Fownes. Source: Wellcome Collection.
98/608 (page 106)
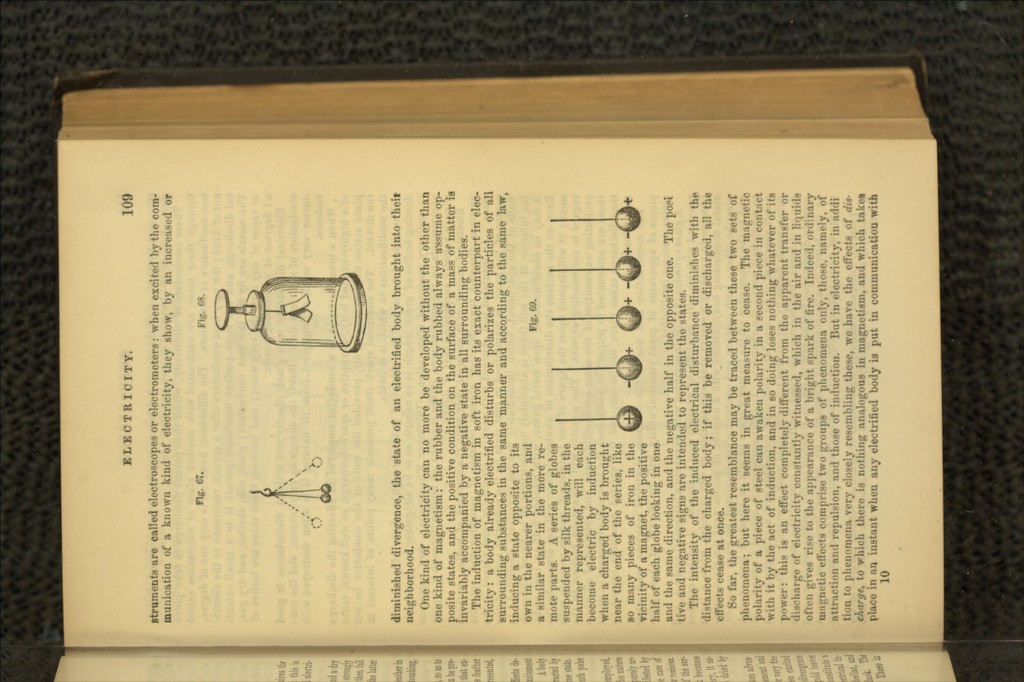
![The whole class of paramagnetic bodies behave in a precise./ similar way under similar circumstances; only in. the intensity of the effects great differences occur. On the contrary, diamagnetic bodies have their long diameters placed equatorially, that is, at right angles to the magnetic axis. They behave, as it' at the end opposite to each pole of the magnet, the same kind of polarity existed. lu the first class of substances, besides iron, which is the best representative of the class, we have nickel, cobalt, manganese, chromium, cerium, titanium, palladium, platinum, osmium, aluminium, oxygen, and also most of the compounds of these bodies; most of them, even when in solution. According to Faraday, the following substances are also feebly paramagnetic (magnetic): paper, sealing-wax, Indian-ink, porcelain, asbestos, fluorspar, minium, cin- nabar, binoxide of lead, sulphate of zinc, tourmaline, graphite, and charcoal. •In the second class are placed bismuth, antimony, zinc, tin, cadmium, sodium, mercury, lead, silver, copper, gold, arsenic, uranium, rhodium, iridium, tungsten, phosphorus, iodine, sulphur, chlorine, hydrogen, and many of their compounds. Also, glass free from iron, water, alcohol, ether, nitric acid, hydrochloric acid, resin, wax, olive oil, oil of turpentine, caoutchouc, MI gar, starch, gum, and wood. These are diamagnetic. If diamagnetic and paramagnetic bodies are combined, their peculiar properties are destroyed. In most of these compounds, occasionally, in consequence of the presence of the smallest quantity of iron, the peculiar magnetic power remains more or less in excess Thus green bottle-glass and many varieties of crown glass, are magnetic in consequence of the iron they contain. In order to examine the magnetic properties of fluids they are placed in very thin glass tubes, the ends of which are then closed by melting; they are are then hung horizontally between the poles of the magnet. Under the influence of poles sufficiently powerful, they begin to swing, and according as the fluid contents are paramagnetic (magnetic), or diamagnetic, they assume an axial or equatorial position. Under certain circumstances substances which belong to the paramagnetic class behave as if they were diamagnetic. This happens in consequence of a differentia] action. Thus, for example, when a glass tube full of a dilute solution of sulphate of iron is allowed to swing in a concentrated solution of sulphate of iron, instead of in the air, it assumes an equatorial position. The air, in consequence of the oxygen in it. is itself paramagnetic (magnetic). J Irnre, such bodies as appear to possess feeble diamagnetic properties, can only show their true properties when hung in a vacuum. Faraday has tried the magnetic condition of gases in different ways. One way consisted in making soap bubbles with the gas which he wished to 'nvestigate, and bringing these near the poles. Soap and water alone is feebly diamagnetic. A bubble filled with oxygen was strongly attracted by the magnet. All other gases in the air are diamagnetic; that i>s, they are repelled. But, as Faraday has shown, in a different way, this partly arises from the paramagnetic (magnetic) property of the air. Thus he found that nitr.'p-n. when this differential action was eliminated, was perfectly indifferent, whether it was condensed or rarefied, whether cooled or heated, When the temperature is raised,.the diamagnetic property of gases in the air is increased, llem-e the flame of a candle or of hydro-ren is strongly repelled by the magnet, Ji\i-n warm air is diamagnetic' in cold air. For .-Dine time it had been believed that bodies in a crystalline form had a ppecia! and peculiar behavior when placed between the poles of a magnet. It appeared as though the magnetic directing power of the crystal had some peculiar relation to the position of its optic axis; so that, independently of the magnetic property of the substance of the crystal, if the crystal wa§](https://iiif.wellcomecollection.org/image/b21183806_0098.jp2/full/800%2C/0/default.jpg)