Introduction to the study of inorganic chemistry / by William Allen Miller.
- Miller, William Allen, 1817-1870.
- Date:
- 1871
Licence: Public Domain Mark
Credit: Introduction to the study of inorganic chemistry / by William Allen Miller. Source: Wellcome Collection.
37/332
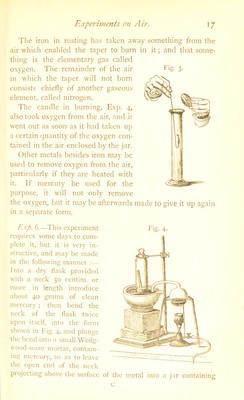
![Fig. The iron in rusting has taken away something from tlie air which enabled the taper to burn in it; and that some- thing is the elementary gas called o.xygen. The remainder of the air in which the taper will not burn consists chiefly of another gaseous element, called nitrogen. The candle in burning, E.\j). 4, also took o.xygen from the air, and it went out as soon as it had taken u]) a certain quantity of the oxygen con- tained in the air enclosed by the jar. Other metals besides iron may be used to remove oxygen from the air, particularly if they are heated with it. If mercury be used for the purpose, it will not only remove the oxygen, but it may be afterwards in a separate form. Rxp. 6.—This experiment requires some days to com- plete it, but it is very in- structive, and may be made in tlie following manner :— Into a dry flask provided with a neck 50 centim. or more in length introduce about 40 grams of clean mercury ; then bend the neck of the flask twice upon itself, into the form shown in Fig. 4, and plunge the bend into a small Wedg- wood-ware mortar, contain- ing mercury, so as to leave the open end of the neck projecting above the surface Fig. 4. of the metal into a jar containing c](https://iiif.wellcomecollection.org/image/b28099631_0037.jp2/full/800%2C/0/default.jpg)