Introduction to the study of inorganic chemistry / by William Allen Miller.
- Miller, William Allen, 1817-1870.
- Date:
- 1871
Licence: Public Domain Mark
Credit: Introduction to the study of inorganic chemistry / by William Allen Miller. Source: Wellcome Collection.
38/332
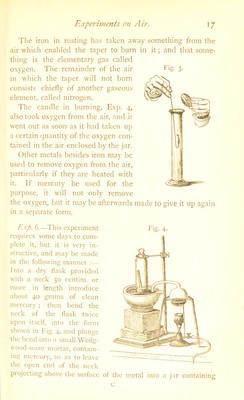
![air, which is to be siipporlccl over it. Now apply the heat of a lamp to the flask, and keep the mercury for two or three days at a point just below that necessary to make it boil. Red scales will be formed slowly upon the surface of the mercury in the flask ; and these scales after a time will no longer increase in quantity. If the lamp be then withdrawn, and the whole allowed to cool, the bulk of the air will be found to have become considerably less. The hot mercury has acted chemically on the air both of the flask and of the jar, owing to the free passage of both ])ortions through the neck. I'he gas which is left consists almost entirely of nitrogen. On adding mercury till the height outside and inside the jar is the same, and thtm withdrawing the stopper and in- troducing a lighted taper, supported on a wire handle, it will be put out. A mouse or other small animal would also soon die if plunged into it. The o.xygen is the portion of the air necessary to support the life of animals. If one or two grams of the red scales formed by thus heating mercury in air be placed in a test tube, they may be made to give up the oxygen again by heating them still more strongly. Exp. 7.—Fit a good cork to the mouth of the tube ; then with- draw the cork, and with a round file bore a hole through it, just large enough to f‘g- 5- admit a narrow glass tube, bent as shown in Fig. 5. Heat the tube and the red scales in the flame of a spirit lamp while the open end of the narrow tube is dipped underwater. Bubbles of gas will soon begin to come off. Next fill two or three narrowjars or wide test tubes with water; close them with the finger, and in- vert them in the basin ; collect the bubbles of gas in one of them as they escape from the narrow tube. The first jar will be filled chiefly with the air originally in the heated tube ; this may be thrown away; but if into one of the other tubes, when filled with](https://iiif.wellcomecollection.org/image/b28099631_0038.jp2/full/800%2C/0/default.jpg)