Introduction to the study of inorganic chemistry / by William Allen Miller.
- Miller, William Allen, 1817-1870.
- Date:
- 1871
Licence: Public Domain Mark
Credit: Introduction to the study of inorganic chemistry / by William Allen Miller. Source: Wellcome Collection.
40/332
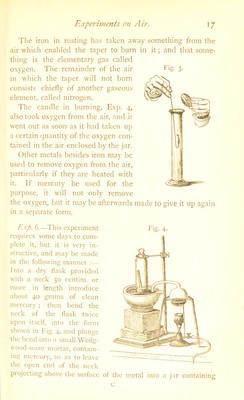
![1)C mixed with about its own weight of black manganese oxide in fine powder. This oxide should be first made red hot in a covered clay crucible, and allowed to cool; it should then be ground up in a clean mortar with the chlorate. The manganese oxide enables the oxygen to pass off from the chlorate at a much lower heat than is needed if the salt is heated alone, although the oxide itself undergoes no permanent change. 30 or 40 grams of this mixture may be put into a clean and dry Florence oil flask, provided with a good cork, through which is passed a tolerably wide bent glass tube. The flask is to be placed with the end of the tube dipping under the water in the pneumatic trough, Fig. 6. If the mixture in the flask is heated over a lamp, gas comes off freely, and may be collected in jars placed for its reception. Fig. 6. i)ncumatic trougli for experiments upon gases may be easily made out of a small tub or ])an, which is to be nearly filled with water. A shelf must be fixed at one end, so as to be 3 or 4 centim. below the surface of the water, or the glass jar may even be supported on a brick; 3 or 4 jar.s, each holding about a litre, may be used to receive the gas. 'J'hey should be open below, and one or two may be provided with a glass stopper ground to fit the neck. I'hcy](https://iiif.wellcomecollection.org/image/b28099631_0040.jp2/full/800%2C/0/default.jpg)