Introduction to the study of inorganic chemistry / by William Allen Miller.
- Miller, William Allen, 1817-1870.
- Date:
- 1871
Licence: Public Domain Mark
Credit: Introduction to the study of inorganic chemistry / by William Allen Miller. Source: Wellcome Collection.
41/332
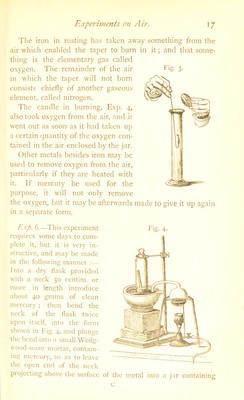
![may be filled with water in the trough, and placed with tlie bottom downwards on the shelf. As they become filled one after another with the gas, they can be removed by sliding a j'late under each, while the mouth is still under water, and then lifting the plate and jar together out of the trough. Though the potassic chlorate is more easily decomposed when mi.\ed with manganese o.xide than when heated alone, the pure salt may be made to give off o.xygen by heating it more strongly by itself Exp. 9.—Place about a gram of the salt in a test lube, and heat it over a spirit lamp. The chlorate snaps and flies to pieces, or decrepitates, when first heated. It then melts and forms a clear liquid, which, when heated more strongly, gives off bubbles of pure oxygen gas. The mass gra- dually becomes white and opaque, and ceases to give off o.xygcn, leaving a white residue, consisting of chlorine and potassium only, and known as potassic chloride. The gas at first often looks cloudy, owing to little particles of the salt which are carried over suspended in it in fine powder, but these gradually become dissolved in the water. 245 grams of the chlorate would give off 96 grams of oxygen, or about 67-2 litres of the ga.s. The change may be thus represented :— Potassic Chlorate Potassic Chloride 2 K Cl O3 = 2K Cl + 3O, 2('39 + 35‘5 + i6x3) 2(39 + 35-5) 6x.i6 v i , > > , - =45 149 96 =45 If the mineral known a.s black manganese oxide (MnO.) Ibe made red hot, oxygen may also be obtained from it; but only one-third of its oxygen is thus driven off, or about one- ninth of the weight of the mineral if jture. The ore f)f manganese, however, always contains im]niritie.s, which cause ' the oxygen gas to be mixed with more or less of other gases. The black oxide when heated becomes converted, with loss](https://iiif.wellcomecollection.org/image/b28099631_0041.jp2/full/800%2C/0/default.jpg)