Copy 1, Volume 1
Hand-book of chemistry / Translated by Henry Watts.
- Gmelin, Leopold, 1788-1853
- Date:
- 1848-1872
Licence: Public Domain Mark
Credit: Hand-book of chemistry / Translated by Henry Watts. Source: Wellcome Collection.
464/562 (page 440)
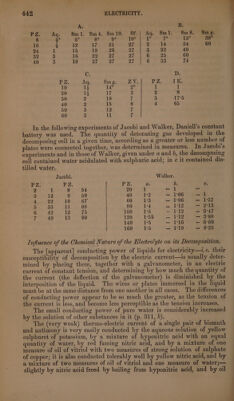
![of iodide of potassium, the other with solution of sulphate of soda, and the circuit is closed by a bent platinum wire, whose ends rest on the two soda, not even when the iodide of potassium paper is removed, and metallic contact established. If one of the platinum plates be replaced by a capsule, in which nitre or chloride of lead is maintained in a state of fusion—the zine and platinum plates being immersed in dilute sulphuric acid—the iodide of potassium is decomposed, but not the nitre or the chloride of lead: chloride of silver, on the contrary, is decomposed as readily as the iodide of potassium. It appears then that. water, solution to currents of very small intensity without being decomposed. (Faraday.) The current from a single pair does not decompose water, and yet produces deflection of the galvanometer. If the current of a ten-pair battery be weakened by the interposition of a long thin wire, to such a degree that it produces the same deflection as the current of a single pair, it will behave towards water in the same manner as the latter. Hence feeble currents pass through water without decomposing it. (Jacobi.) It-is true that no gas is obtained when a feeble current is conducted into a watery liquid by means of platinum plates: nevertheless, the water is decomposed—but so slowly, that the oxygen and hydrogen gases liberated on so large a surface partly surround it as an envelope, and are partly absorbed by the water. Even when two fine platinum wires are used as electrodes, not a single bubble of gas escapes. But if one elec- trode consists of a platinum plate, the other of a fine wire of the same metal covered with glass up to its point, according to Wollaston’s method (p. 438), gas is evolved at the point of the wire—though only for a short time—when the water is subjected to the action of a pair of plates of zinc and platinum in solution of common salt. The evolution of gas is renewed, both when the surface of the larger electrode is increased, and like- wise when the current is reversed. The gas appears at the narrow elec- trode, because the wider surface of the other greatly facilitates absorption. When the electrodes have equal surfaces, the positive electrode becomes negative, in consequence of a film of oxygen attached to it—and the nega- tive electrode positive, from a film of hydrogen; and thus the primitive current is weakened (vid. Secondary Currents), so that the water is de- composed at the same rate only as absorption goes on.—Sulphate of soda may also be decomposed by the current of a pair of zinc and platinum in solution of common salt, by means of electrodes of very unequal surface like those above described. (Andrews, Pogg. 41, 166.)—Grove also (Pogg. 48, 305) concludes from his experiments, that water does not conduct the electric current without undergoing decomposition. poses iodide of potassium, does not [visibly] decompose acidulated water or solution of nitrate of silver. The two last mentioned liquids are not decomposed even when the plates are immersed in dilute sulphuric acid; but when a small quantity of nitric acid is added to the liquid in the zinc and copper, having the surface of a square metre and immersed in dilute sulphuric acid, without any nitric acid, likewise decomposes nitrate](https://iiif.wellcomecollection.org/image/b33289190_0001_0464.jp2/full/800%2C/0/default.jpg)