Copy 1, Volume 1
Hand-book of chemistry / Translated by Henry Watts.
- Gmelin, Leopold, 1788-1853
- Date:
- 1848-1872
Licence: Public Domain Mark
Credit: Hand-book of chemistry / Translated by Henry Watts. Source: Wellcome Collection.
465/562 (page 441)
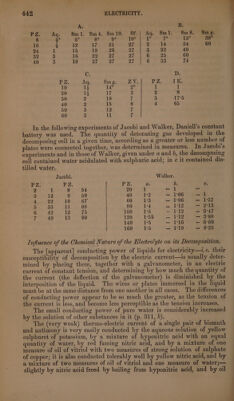
![city. Hence decomposition depends only on the quantity of electricity. (Matteucci.) [That however the intensity of the current exerts great influence, is evident from the fact that combination of a number of pairs of plates however small will decompose any electrolyte whatever. | If the electrodes, dipping into acidulated water, consist of one of the more oxidable metals instead of platinum [in which case the affinity of © those metals for oxygen will favour the decomposition] then, according to Henrici, the current of a single pair consisting of zine, dilute sulphuric acid, sulphate of copper, and copper, will decompose water and evolve hydrogen gas, the deflection of the galvanometer increasing as follows, with the quantity of gas obtained in an hour: Platinum. Silver. Copper. Brass. _ Steel. Tin. Zine. Cub. centim. Hydrogen gas 0 0°3 12 19 34 36 72 sine Of angie.of. deflection, -.0° 1’; 07.6’. ..0°:8' . 0°.18°. 8°26’. 9° Il’. 38°17? Some decomposition of water is doubtless effected with platinum electrodes, but as the oxygen evolved does not combine with the platinum the action is retarded. (Henrici.) | To this head likewise belong the experiments mentioned, pp. 403....408. _ Thermo-electric currents, weak both in quantity and intensity, decom- pose nitrate of silver, when the electrodes consist of platinum,—but not salts of copper, lead, tin, or zinc; in these also electrodes of gold or silver produce no effect. But when the electrodes consist of the same metal as that in the solution, decomposition is easily effected—e. g. nitrate of silver - with silver electrodes, sulphate of copper with copper, protochloride of tin with tin, acetate of lead with leaden electrodes. Platinum wires in solutions of platinum produce no effect. A thermo-electrie current is best conducted into a solution of common salt by means of wires of zine, tin, lead, or iron,—less readily by copper, with difficulty by silver, and not at all by platinum. Similar to the action of the thermo-electric current is that of the very feeble current obained when the vessel a (App. 2) contains potash, 6 nitric acid, and the asbestus fibres / solution of common salt—platinum wires dipping into a and 0, and being connected with wires of the metals above mentioned. (Becquerel.) [In this case, the electricity has no affinity to overcome—because, for every portion of metal separated at the cathode, an equal portion combines with oxygen and acid at the anode, so that the electricity has only to transpose the atoms. | | The following experiments were made to determine the proportion in which the decomposition of different liquids,—and therefore also the quantity of the current, increases with the number of pairs: : In A a pile was constructed of zinc and copper with solution of sal- ammoniac, in B with water containing nitro-sulphuric acid, and in C with spring-water; in D a battery of zinc and platinum in dilute sulphuric acid was employed.—The liquid in the decomposing cell was water = Aq , or a solution of 1, 4, 8, or 10 parts of sal-ammoniac in 100 parts of water = Sm 1, Sm 4, Sm 8, Sm 10; or saturated solution of sal-ammoniac=Sm g; or dilute sulphuric acid = Sf; ora solution of 1 part of sulphate of zine (zinc-vitriol) in 100 parts of water = Z V; or solution of iodide of potas- sium =IK.—PZ denotes the number of pairs employed.—The degrees. under A, B, C give the deflection of the galvanometer; the numbers under D denote the relative quantity of hydrogen gas evolved from the solution — of iodide of potassium. (Matteucci.)](https://iiif.wellcomecollection.org/image/b33289190_0001_0465.jp2/full/800%2C/0/default.jpg)