Copy 1, Volume 1
Hand-book of chemistry / Translated by Henry Watts.
- Gmelin, Leopold, 1788-1853
- Date:
- 1848-1872
Licence: Public Domain Mark
Credit: Hand-book of chemistry / Translated by Henry Watts. Source: Wellcome Collection.
466/562 (page 442)
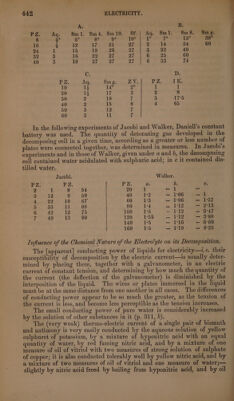
![| A. 3 B. | PZ: Aq. Sm1. Sm4. Smi0. Sf.| Aq. Smil. Sm8. Smg. 8 Ges ge REND SQ Rae EGET phe ae o7s i eg naa 16 4 12 17 21 27 Z 14 34 60 24 1 15 19 26 ya | 3 22 40 32 3 16 22 27 27 6 25 60 40 3 18 27 27 27 6 33 74 C. D. PZ. Aq. Sm g. ZY. PZ. IK; 10 1¢ 14° = 1 1 20 13 17 3 2 8 30 2 18 7 3 17°5 40 2 15 8 4 65 50 3 12 7 60 3 11 7 In the following experiments of Jacobi and Walker, Daniell’s constant battery was used. The quantity of detonating gas developed in the decomposing cell in a given time, according as a greater or less number of plates were connected together, was determined in measures. In Jacobi’s experiments and in those of Walker, given under @ and 0, the decomposing cell coutained water acidulated with sulphuric acid; in ¢ it contained dis- tilled water. Jacobi. Walker. PZ. PZ. 2: a. b. Cc. 4 1 8 54 20 1 — ] — 3 12 9 59 40 12 — 1:06 | 4 22 10 67 60 1-3 =~ 1°06 — 1°57 5 33 11 68 80 1°4 cw ck he — 2°13 6 42 iZ 75 100 1b — 1:12 wm 47 7 49 13 80 120 1°55 — PH aaa 140 1°5 — 1°16 — 6°60 160 | ie aa S19 = 8°25 Influence of the Chemical Nature of the Electrolyte on its Decomposition. The [apparent] conducting power of liquids for electricity—.e, their susceptibility of decomposition by the electric current—is usually deter- mined by placing them, together with a galvanometer, in an electric current of constant tension, and determining by how much the quantity of the current (the deflection of the galvanometer) is diminished by the interposition of the liquid. The wires or plates immersed in the liquid must be at the same distance from one another in all cases. The differences of conducting power appear to be so much the greater, as the tension of the current is less, and become less perceptible as the tension increases. The small conducting power of pure water is considerably increased by the solution of other substances in it (p. 311, 6). The (very weak) thermo-electric current of a single pair of bismuth and antimony is very easily conducted by the aqueous solution of yellow sulphuret of potassium, by a mixture of hyponitric acid with an equal quantity of water, by red fuming nitric acid, and by a mixture of one measure of oil of vitriol with two measures of strong solution of sulphate of copper; it is also conducted tolerably well by yellow nitric acid, and by a mixture of two measures of oil of vitriol and one measure of water;— slightly by nitric acid freed by boiling from hyponitric acid, and by oil ee](https://iiif.wellcomecollection.org/image/b33289190_0001_0466.jp2/full/800%2C/0/default.jpg)