Copy 1, Volume 1
Hand-book of chemistry / Translated by Henry Watts.
- Gmelin, Leopold, 1788-1853
- Date:
- 1848-1872
Licence: Public Domain Mark
Credit: Hand-book of chemistry / Translated by Henry Watts. Source: Wellcome Collection.
487/562 (page 463)
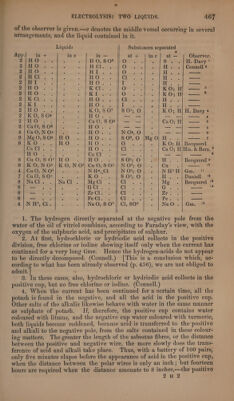
![yor sition of atoms in the liquids of the body situated between the electrodes; and since in particular organs, salts may be thus produced different from those previously existing, the result of the action may be irritation. The first of these modes of action is most conspicuous in electricity of great intensity, like that of the machine,—the second in galvanic elec- tricity: and hence, perhaps, partly arises the difference between the sen- sations produced by these two kinds of electricity. Even a single pair of metallic plates connected with each other, and with different parts of the body, must produce transposition of atoms and change of composition— and this is the principal cause of the phenomena observed by Galvani and Humboldt. Oxygen-Salts of Heavy Metallic Oxrdes. The compounds of oxygen-acids with heavy metallic oxides usually give acid and oxygen gas at the anode, and reduced metal at the cathode, —no evolution of hydrogen taking place, excepting when the electric cur- rent is too strong, and its action is partly exerted on the water. In the case of some metallic salts (those of manganese, lead, and silver), the oxygen liberated at the positive pole combines with the metallic oxide there situated, and forms with it a peroxide which is precipitated. If the electrodes are formed of the same metal as that contained in the solution, the acid and oxygen which collect at the anode recover by dissolving it the metal which they have lost, and reproduce the original salt; thus the liquid remains unaltered, and the anode merely loses a quantity of metal equal to that which is reduced at the cathode. Salts of Manganese deposit peroxide at the positive pole. (Balard.) Sulphate of Zine dissolved in water yields zinc at the negative pole, in the form of a powder possessing the metallic lustre. Fused Borate of Lead yields lead at the cathode, oxygen and boracic acid at the anode. (H. Davy.) Aqueous solution of Vitrate or Acetate of Lead yields lead at the nega- tive pole, and brown peroxide of lead at the positive pole. (Faraday.)— If a voltameter be introduced into the circuit, it is found that the volume of oxygen gas evolved at the anode from solution of acetate of lead amounts to only +1, of the detonating gas collected in the voltameter,— because the greater part of the oxygen is expended in forming the peroxide of lead, the quantity of which is to that of the lead separated at the cathode as 5 : 3[?].—When the current is passed through acetate of lead which has been dehydrated and then fused, peroxide of lead is likewise produced at the anode, accompanied by a slight evolution of meter there is obtained at the cathode 103°4 parts (1 atom) of lead, and the weight of this lead is to that of the peroxide produced as 3: 5 [?] (Matteucci, Ann. Chim. Phys. 71, 90.) From a solution of Protosulphate of Iron, acted upon by a battery of 100 pairs, metallic iron is deposited in small granules on the positive pla- tinum wire. (Becquerel.) In solution of Sulphate of Copper, the negative platinum wire becomes covered with copper, while acid collects and oxygen gas is evolved at the positive wire. When copper wires are used, the positive wire loses as much metal as the negative wite gains, and the solution retains its original composition. When active iron forms the anode in solution of sulphate of copper, it](https://iiif.wellcomecollection.org/image/b33289190_0001_0487.jp2/full/800%2C/0/default.jpg)