Copy 1, Volume 1
Hand-book of chemistry / Translated by Henry Watts.
- Gmelin, Leopold, 1788-1853
- Date:
- 1848-1872
Licence: Public Domain Mark
Credit: Hand-book of chemistry / Translated by Henry Watts. Source: Wellcome Collection.
491/562 (page 467)
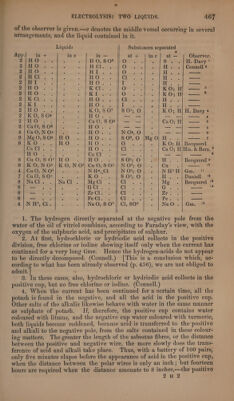
![A67 Liquids Substances separated App. in + ine in — at + ine} at — Observer. B PEDO aire HO, 8Q% O°. pity Seay H. Davy ' BidtiOiy. HCl... % O - | H. .« | Connell 2 i oS Bh Petit O rere es epee Sat wi. HU. os Cl - |H..}| — 2 2 ea pl a 1 ema »- |A..} — Ym, me Congo, ges . |KO; H| — ? is be Rilasesrt 0 02s . |KO; HH} — % MR Clls f« ‘ 8 ree Chen > |...) me Fe S. Se : HO. 43% ere cviky Bot’ AR ee ae ‘ K O, SO? || SO3; O - | KO; H/ H. Davy 4 2/KO, S03 A HUD. 5 —_- — : -— — 4 hd es Sh lari e Ca 0, S 03 —_- — : CaO; H} —— 5 21CaO,SO8 |] . HO. 3 _- — i — —| — is 4/CaO,NO5|] . HiOte: ss N 05, O : — —| — 6 9 |Mg0,SO3;HO HO. so0?,0 MgO}H. . 7 iG Meee RE on adobe Ce) OD... 24. } 5&0; EL} Becquerel Ca Cl : : 15 G8 hae 1 oa Ca O; H|His. & Berz. ® HO 7 oT we sec iiatetape —- — ® $;/CuO0O;S 07-HO . HO. S$ 03; O H. Becquerel 9 8|K O,NO5/} KO, NO Cu O,8S 03 || NO; O Cals 1g 4;Cu0,NO*! . . | NH4, Cl NO; O NH? H|Gm. ?! 7/CuO,SO3]. . KO S 0°; O is eG Daniell 12 8 | NaCl Na Cl Mg Cl 2 re Mg Becquerel 1% 8} — . ‘ GCl. et Bide iyo: a 8}; — Zr Ci. 2 beat 5 ale sao a Fe Cl. Cli « oe Pe fe a 4.N Ht, Cl; NaO, S O? |) Cl, SO° NaO.|Gm. 1. The hydrogen directly separated at the negative pole from the water of the oil of vitriol combines, according to Faraday’s view, with the oxygen of the sulphuric acid, and precipitates of sulphur. 2. At first, hydrochloric or hydriodic acid collects in the positive division, free chlorine or iodine showing itself only when the current has continued for a very long time. Hence the hydrogen-acids do not appear to be directly decomposed. (Connell.) [This is a conclusion which, ac- cording to what has been already observed (p. 456), we are not obliged to admit. “t 3. In these cases, also, hydrochloric or hydriodic acid collects in the positive cup, but no free chlorine or iodine. (Connell.) 4, When the current has been continued for a certain time, all the potash is found in the negative, and all the acid in the positive cup. Other salts of the alkalis likewise behave with water in the same manner as sulphate of potash. If, therefore, the positive cup contains water coloured with litmus, and the negative cup water coloured with turmeric, both liquids become reddened, because acid is transferred to the positive and alkali to the negative pole, from the salts contained in these colour- ing matters. The greater the length of the asbestus fibres, or the distance between the positive and negative wire, the more slowly does the trans- ference of acid and alkali take place. Thus, with a battery of 100 pairs, only five minutes elapse before the appearance of acid in the positive cup, when the distance between the polar wires is only an inch; but fourteen hours are required when the distance amounts to 8 inches,—the positive](https://iiif.wellcomecollection.org/image/b33289190_0001_0491.jp2/full/800%2C/0/default.jpg)