Copy 1, Volume 1
Hand-book of chemistry / Translated by Henry Watts.
- Gmelin, Leopold, 1788-1853
- Date:
- 1848-1872
Licence: Public Domain Mark
Credit: Hand-book of chemistry / Translated by Henry Watts. Source: Wellcome Collection.
79/562 (page 55)
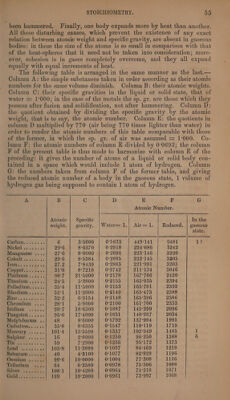
![been hammered. Finally, one body expands more by heat than another. All these disturbing causes, which prevent the existence of any exact relation between atomic weight and specific gravity, are absent in gaseous bodies: in these the size of the atoms is so small in comparison with that of the heat-spheres that it need not be taken into consideration; more- over, cohesion is in gases completely overcome, and they all expand equally with equal increments of heat. The following table is arranged in the same manner as the last.— Column A: the simple substances taken in order according as their atomic numbers for the same volume diminish. Column B: their atomic weights. Column C: their specific gravities in the liquid or solid state, that of water = 1'000; in the case of the metals the sp. gr. are those which they possess after fusion and solidification, not after hammering. Column D: the quotient obtained by dividing the specific gravity by the atomic weight, that is to say, the atomic number. Column E: the quotients in column D multiplied by 770 (air being 770 times lighter than water) in order to render the atomic numbers of this table comparable with those of the former, in which the sp. gr. of air was assumed = 1:000. Co- lumn F; the atomic numbers of column E divided by 0:0693; the column F of the present table is thus made to harmonize with column E of the preceding: it gives the number of atoms of a liquid or solid body con- tained in a space which would include 1 atom of hydrogen. Column G: the numbers taken from column F of the former table, and giving the reduced atomic number of a body in the gaseous state, 1 volume of hydrogen gas being supposed to contain 1 atom of hydrogen. A B C D E F G Atomic Number. Atomic | Specific In the weight. | gravity. |Water=1.| Air=1. | Reduced. | gaseous state. Carbon........ 6 3°5000 0°5833 449°14] 6481 1? Wickel si .as.ss | 29°6 8°6370 0:2918 224°686 3242 Manganese .... 27°6 80000 0°2898 223°146 3220 Sieualt seve a.s.| ° 29-6 8°5384 0°2885 222°145 3205 BPOst bh cuts cg] . 27.2 7°8439 0°2883 221°991 3203 Depperii.ss...) . 31°S 8°7210 0°2742 211°134 3046 Platinum....../ 98°7 21°5000 0°2178 167°706 2420 Titanium ...... 24°5 5°2800 0°2155 165°935 2394 Palladium..i...| 35°4 11°5000 0°2153 165°781 2392 Rhodium...s:.| 52:1 11-2000 0°2149 165°473 2388 MING. aan wae es], 32°2 6°9154 0°2148 165°396 2386 Chromium ....| 28:1 5°9000 0°2100 161°700 2333 rium shisi.} (98-7 18-6300 0°1887 145°299 2096 Tungsten ...... 95°0 17°4000 0°1831 140°987 2034 Molybdenum .. 48 8°6000 0°1792 137°984 1991 Cadmium... i: 55°8 8°6355 0°1547 119°119 1719 Mercury ......| 101°4 13°5590 0°1337 102°949 1485 J Sulphur... .... 16 2°0000 0°1250 96°250 1388 6 MimAteeiieit ss) 59 7°2900 0°1236 95°172 1373 Lead ...iss.55{ 103°8 11°3889 0°1097 84°469 1218 Selenium ...... 40 4°3100 0°1077 82°929 1196 Osmium ......| 99°6 10°0000 0°1004 77°308 1116 Tellurium .... 64 6°2580 0°0978 75°306 1088 myer cur. .ec.. | 108-1 10°4280 0°0964 74°218 1071 MUM vigsestee| 199 19°2000 0°0961 73°997 1068](https://iiif.wellcomecollection.org/image/b33289190_0001_0079.jp2/full/800%2C/0/default.jpg)