Copy 1, Volume 1
Hand-book of chemistry / Translated by Henry Watts.
- Gmelin, Leopold, 1788-1853
- Date:
- 1848-1872
Licence: Public Domain Mark
Credit: Hand-book of chemistry / Translated by Henry Watts. Source: Wellcome Collection.
84/562 (page 60)
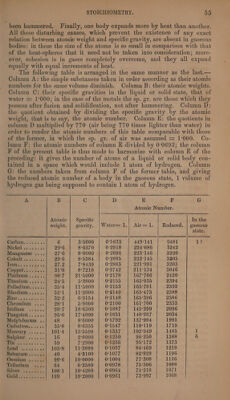
![that when the proximate elements vf such compounds of the second order, sulphate of lead for example, contain a common ultimate ele- ment, as oxygen in this case, the quantities of this ultimate element con- tained in the two proximate elements must bear a simple relation to each other: ¢.g., the quantity of oxygen in sulphuric acid is exactly 3 times as great as that in the oxide of lead combined with it. Chemical Formule. A chemical formula is an expression by symbols and numbers of the composition of a definite chemical compound according to its elements and their relative quantities. The symbols are the initial letters of the names of the elementary substances given in the table, page 50, column B. Certain compounds, particularly of the organic class, have likewise particular symbols appropriated to them: e. g., Water = Aq; Cyanogen = Cy; Tartaric acid = T; Citric acid = C; Acetic acid = A; Quinine + + = Ch; Morphia = M, &c. The numbers annexed to the symbols denote the numbers of atoms of the several constituents existing in the com- pound; a symbol with no number annexed to it implies that one atom only of the corresponding substance exists in the compound. Electro- positive substances, such as metals and salifiable bases, precede electro- negative substances, such as oxygen and acids in the formule. This order is the reverse of that adopted in the nomenclature, but it would perhaps be better in this as well as in the formule to give precedence to the electro-positive element. When a compound contains proximate and ultimate elements, the mode of combination is expressed by means of points, commas, + signs, and brackets. Oxygen, which occurs so fre- quently in compounds, is often expressed by points placed over the sym- bol of the body with which it is in combination, the number of these points being equal to the number of atoms of oxygen present. In a simi- lar manner, strokes leaning from right to left are used to denote atoms of sulphur, and points under the symbol of the other body, atoms of hydrogen. Oxide of lead is PhO = Pb; potash (1 At. potassium and ] At. Oxy- gen) is KO = K; water is HO = H; alumina (2 At. aluminum and 8 At. oxygen) is Al? 0? = Al; carbonic acid is CO? = C; silica is Si O? = Si; sulphuric acid is SO? = §; nitric acid is NO’ = N ; ammonia is NH? = N; sulphate of lead is PhO + SO* = PbO, SO = Pb §; bicarbonate of potash (1 At. potash, 2 At. carbonic acid, and 1 At. water) is KO +. 2CO? + HO = KO, CO*, HO = KC? Hj; crystallized sulphate of ammo-. nia (1 At. ammonia, 1 At. sulphuric acid, and 2 At. water) is NH? + SO? + 2HO = NH, 80°, 2HO, = NS H?; crystallized potash alum (1 At. potash, 1 At. alumina, 4 At. sulphuric acid, and 24 At. water) is (KO + SO’) + (Al O? + 380°) + (24 HO) = KO, SO? + ALP 0%, 3803 +24HO=K S, Al S§, H; sulphuret of potassium (1 At. potassium and 1 At. sulphur) is KS = K; tersulphuret of molybdenum is MoS? = Mo ; the combination of these two metallic sulphurets in equal numbers](https://iiif.wellcomecollection.org/image/b33289190_0001_0084.jp2/full/800%2C/0/default.jpg)