Copy 1, Volume 1
Hand-book of chemistry / Translated by Henry Watts.
- Gmelin, Leopold, 1788-1853
- Date:
- 1848-1872
Licence: Public Domain Mark
Credit: Hand-book of chemistry / Translated by Henry Watts. Source: Wellcome Collection.
92/562 (page 68)
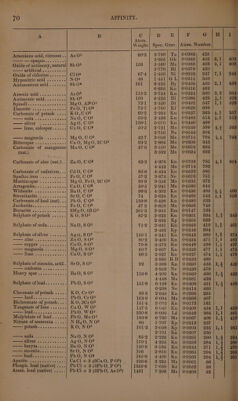
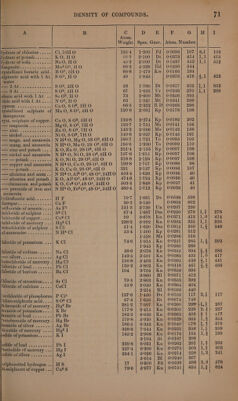
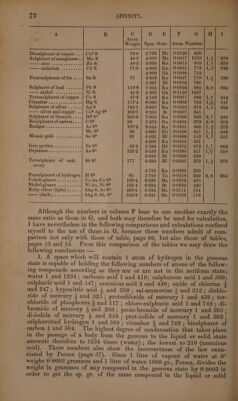
![} ts liquid bodies takes place without alteration of volume. In most cases condensation ensues, very rarely expansion; a very slight expansion takes place in the combination of iodine with potassium, lead, mercury, or silver, of sulphur with arsenic (in the formation of the red sulphuret) with copper (in the formation of disulphuret of copper) or with cadmium, (Karsten, Boullay,) and of lead with tin, gold, or platinum (Kupffer) ; also a considerable expansion in the combination of carbon (of 3°5 sp. gr.) with sulphur (of 2:0 sp. gr.) to form bisulphuret of carbon (of 1:272 sp. gr.). But these expansions or contractions exhibit, as might be ex- pected from the reasons stated on page 54, no such simple relation as that which is observed in all cases of the combination of gases. That some approach to a definite relation does however exist between the density of elements and that of their combinations in the solid or liquid state, appears from the labours of Le Royer and Dumas, Herapath (Phil. Mag. 1824, Nov. 321; abstr. NV. Zr. 11, 164), Pol. Boullay (Ann. Cham. Phys. 48, 266, also Pogg. 19, 107, also WV. 7'r. 23, 1, 208), Karsten, Ammermiiller, H. Kopp, and especially of H. Schroder. These relations are exhibited in the following table, which, however, is drawn up, not according to the Theory of Volumes of Kopp and Schroder, but according to the Atomic Theory. Column A. Name of compound.—B. Formula. ©. Atomic weight.—D. Specific gravity in the liquid or solid state, that of water = 1. EK. Name of the observer of the specific gravity. Bg. denotes Bergman ; Bk. Brooke ; Bl. Boullay ; Bt. Breithaupt ; Bu. Bucholz; By. Bussy ; Bz. Berzelius; Dm. Dumas; Dt. Dalton; Dv. Davy; Ek. Eckeberg ; Fd. Faraday; Gb. Guibourt; GL. Gay-Lussac; Gk. Glocker; Hd. Haidinger ; Hf. Hoffmann; Hr. Herapath; Ke. Kersten; Kf. Kupffer 5 Kn. Karsten; Kp. Kopp; Kw. Kirwan; Ld. Leonhard; Ms. Mohs; Mt. Mitscherlich; Ne. Neumann; Ni. Niemann; Ph. Phillips; RD. Le Royer and Dumas ; Rm. Rammelsberg ; Rt. Regnault ; St. Stromeyer ; Ta. Taylor; Te. Thénard; Ti. Thilorier; To. Thomson ; Un. Unver- dorben ; Ur. Ure; W. Wohler. F. Quotient obtained by dividing the sp. gr. by the atomic weight ; this column is therefore comparable with column D of table, page 55. G. Numbers in column F multiplied by 770 and then divided by 0:0693 for reasons explained on page 55. Hence the numbers in column G show the number of atoms of a compound included in the space which would contain one atom of hydrogen in the state of gas. Thus then the numbers in column G are comparable with those of column E of table, page 53, as well as those in column F of table, p. 55, and those of column I of table, page 66. For explanation of columns H and I see page 75. A B C D E F Gi ty ll Atom. Weight.| Spec. Grav. | Atom. Number. Suboxide of copper...... Cuz0 71:6 | 5-300} Bl | 0°0740| 822 |2,2| 883 5°751 | Kn | 0°0803] 892 6°093 | Hr | 00851} 9465 Suboxide of mercury....| Hg? O 210°8 | 8:950| Kn | 0°0425| 472 |6,1]| 475 10°690 | Hr | 0°0507| 563 WiRiOE <2 ews cose 2's ILO 9 1°000 K 0-1111| 1234 | 1,1} 1234 Pedece fot. ce oS KO 472 | 2°656}°* | 0:0563} 625 |1,4| 625 BG Heel ln dalee ae siete he NaG 31:2 | 2°805| Kn | 0:0899| 999 |1,41 1048 avyte see hada t Ba O 766 | 4°732| Kn| 0:0618| 687](https://iiif.wellcomecollection.org/image/b33289190_0001_0092.jp2/full/800%2C/0/default.jpg)