Copy 1, Volume 1
Hand-book of chemistry / Translated by Henry Watts.
- Gmelin, Leopold, 1788-1853
- Date:
- 1848-1872
Licence: Public Domain Mark
Credit: Hand-book of chemistry / Translated by Henry Watts. Source: Wellcome Collection.
96/562 (page 72)
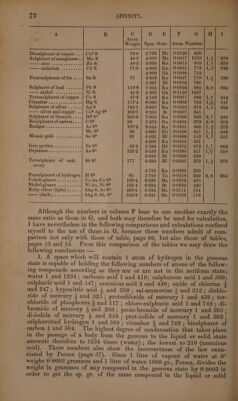
![A B CG | D.dhUECL. FB. eee Weight.| Spec. Grav. | Atom. Number, Di-sulphuret of copper ...| Cu?S Hr | 0°0728; 809 ; Sulphuret of manganese..| Mn S Ms | 0°0917| 1019 | 1,1] 970 Zines, nc ga cuahon an toe Zn8 Kn | 0:0813} 903 }1,1] 878 cadmium .<..s 2. Cd8 Kn | 0:0641) 712 /1,1} 768 Bk | 0:0668| 742 Protosulphuret of tin . Sn 8 Kn | 0:0647| 719 |1,3] 789 Bl | 0:0702| 780 Sulphuret of lead ....... Pbs Kn | 0°0626] 695 |2,0] 694 NICKEL» cee cancun NiS Gk | 071140} 1267 Protosulphuret of copper .} CuS Kn | 0°0871| 968/1,1] 954 maar wa acts s HRS HgS$ Kn | 0°0686} 762 |1,%] 757 Sulphuret of silver ......} AgS Kn | 0°0552| 613 /1,1} 604 silver and eopper ..| Cu® Ag S? St | 0°0307} 341 Sulphuret of bismuth ....] Bi? S3 Kn | 0°0268| 298 |2,1] 295 Bisulphuret of carbon... .} CS? Bz | 0°0335| 372 |2,8| 3873 ON eee eee As S? Kn | 00331] 368 /1,2] 272 Mo S? Hf | 00586] 651 |1,5| 642 Mosaic gold ..... ioves Sn S2 Bl | 0°0488| 542 |1,%} 561 Kn | 0°0505| 561 PEO OUTINGS oc a ov 5 «0 0% Fe S? Hf | 0:0875| 972 /1, 964 POPPI. Gs eae oe ss one As $$ Kn | 0°0280] 311 /1,4] 310 Bl | 0:0296} 329 Tersulphuret of anti- | SbS° Bl | 0°0245) 272 41,8] 276 mony Kn | 0:0269} 299 Persulphuret of bydrogen| H $5 Te | 90-0218} 242 |6,6] 235 Cobalt-glance .......... Co As, Co S? Ms | 0:0378| 420 Nickel-glance .......... Ni As, Ni S? Bt | 0°0378| 420 Ruby silver (light) ...... 3Ag 58, As 89 Ms} 00111} 124 (dark}..........+.| 3AgS, Sb, $3 Ms | 0:0106| 118 Although the numbers in column F bear to one another exactly the same ratio as those in G, and both may therefore be used for calculation, I have nevertheless in the following comparisons and calculations confined myself to the use of those in G, because these numbers admit of com- parison not only with those of table, page 66, but also those of tables, _ pages 53 and 55. From this comparison of the tables we may draw the following conclusions :— 1. A space which will contain 1 atom of hydrogen in the gaseous state is capable of holding the following numbers of atoms of the follow- ing compounds according as they are or are not in the aeriform state; water 1 and 1234 ; carbonic acid 1 and 419; sulphurous acid 1 and 500; sulphuric acid 1 and 547; arsenious acid 2 and 420; oxide of chlorine 4 and 247 ; hyponitric acid 4 and 350; sal-ammoniac + and 312; dichlo- ride of mercury 3 and 325; protochloride of mercury 1 and 439 ; ter- chloride of phosphorus 3 and 117 ; chloro-sulphurie acid 1 and 749; di- bromide of mercury 3 and 289; proto-bromide of mercury 1 and 365 ; di-iodide of mercury % and 258 ; prot-iodide of mercury 1 and 303; sulphuretted hydrogen 1 and 588; cinnabar 2 and 762 ; bisulphuret of carbon 1 and 504. The highest degree of condensation that takes place in the passage of a body from the gaseous to the liquid or solid state amounts therefore to 1234 times (water) ; the lowest to 210 (arsenious acid), These numbers also show the incorrectness of the law enun-~ ciated by Persoz (page 57). Since 1 litre of vapour of water at 0° weighs 0°8003 grammes and 1 litre of water 1000 gr., Persoz, divides the weight in grammes of any compound in the gaseous state by 0°8003 in order to get the sp. gr. of the same compound in the liquid or solid](https://iiif.wellcomecollection.org/image/b33289190_0001_0096.jp2/full/800%2C/0/default.jpg)