A treatise on chemistry. Vol. 1, The non-metallic elements / by Sir H.E. Roscoe & C. Schorlemmer ...
- Henry Enfield Roscoe
- Date:
- 1881
Licence: Public Domain Mark
Credit: A treatise on chemistry. Vol. 1, The non-metallic elements / by Sir H.E. Roscoe & C. Schorlemmer ... Source: Wellcome Collection.
Provider: This material has been provided by University of Bristol Library. The original may be consulted at University of Bristol Library.
205/792 (page 189)
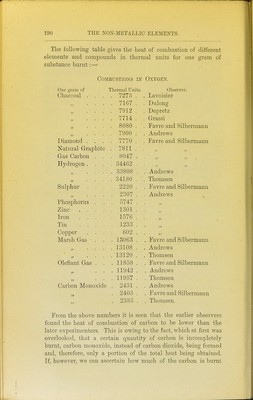
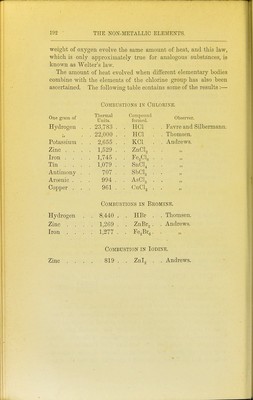
![95 Heat of Comhustion.— It has been shown by numerous ex- periments that when the same weight of the same substance burns to form the same products of combustion, a constant amount of heat is invariably evolved, whether the combustion takes place slowly or quickly. Thus when two parts by weight of hydrogen combine with 1596 parts by weight of oxygen to form 17'96 parts of water, the quantity of heat which is liberated is sufficient to heat 68,924 parts by weight of water from 0° to 1° at what- ever rate the combustion occurs. Thus, too, 11'97 parts by weight of carbon unite with 31'92 parts of oxygen to form 43-89 parts of carbon dioxide, and in this act of union the quantity of heat emitted is sufficient to raise 96,960 parts of water from 0° to 1°. In like manner, the same amount of heat is always set free when the same weight of iron is oxidised, whether this takes place slowly by rusting in the air, or quickly by combustion in oxygen provided, of course, the same oxide be formed in both cases. Since the time of Lavoisier many chemists have measured the amount of heat evolved when different elements combine together. These determinations are surrounded by numerous difficulties, and the most accurate measurements have been made by Favre and Silbermann,^ Andrews,^ and Julius Thomsen.^ ^ Ann. Chim. Phys. [3] xxxiv. 357, xxxvi. 5, xxxvii. 405. 2 Phil. Mag. [3] xxxii. 321, 392, and 426. 3 Ber. Deutsch. Chem. Oes. vi. 1553. ■](https://iiif.wellcomecollection.org/image/b21449016_0205.jp2/full/800%2C/0/default.jpg)