A treatise on chemistry. Vol. 1, The non-metallic elements / by Sir H.E. Roscoe & C. Schorlemmer ...
- Henry Enfield Roscoe
- Date:
- 1881
Licence: Public Domain Mark
Credit: A treatise on chemistry. Vol. 1, The non-metallic elements / by Sir H.E. Roscoe & C. Schorlemmer ... Source: Wellcome Collection.
Provider: This material has been provided by University of Bristol Library. The original may be consulted at University of Bristol Library.
718/792 (page 702)
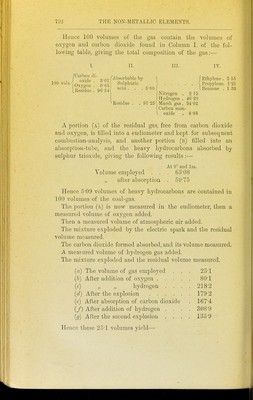
![Hence 100 volumes of the gas contain the volumes of oxygen and carbon dioxide found in Column I. of the fol- lowing table, giving the total composition of the gas:— I. II. in. IV. ^'n'Slp'^'' q-m (Absorbable by ] f Ethylene . 2 55 100 vols. • Sulphuric =^ Propylene. 1-21 Residue: 96-34 ^'^^ ' ' -''''L., j Ben.ene . 1 33 ^ /Nitrogen . 2 15 Hydrogen . 46-20 ^Residue . . 91-25 Ularsh gas . 34-02 Carbon mon- ^ oxide . 8-88 A portion (a) of the residual g-as, free from carbon dioxide and oxygen, is filled into a eudiometer and kept for subsequent combustion-analysis, and another portion (b) filled into an absorption-tube, and the heavy hydrocarbons absorbed by sulphur trioxide, giving the following results :— At 0° and Im. . Volume employed . . . 63'08 „ after absorption . 5975 Hence 5'09 volumes of heavy hydrocarbons are contained in 100 volumes of the coal-gas. The portion (a) is now measured in the eudiometer, then a measured volume of oxygen added. Then a measured volume of atmospheric air added. The mixture exploded by the electric spark and tlie residual volume measured. The carbon dioxide formed absorbed, and its volume measured. A measured volume of hydrogen gas added. The mixture exploded and the residual volume measured. (a) The volume of gas employed . . . 251 (h) AftcT addition of oxygen .... 80-1 (c) „ „ hydrogen . . . . 218-2 (d) After the explosion 179-2 (e) After absorption of carbon dioxide . 167-4 (/) After addition of hydrogen . . . . 308-9 (g) After the second explosion . . . . 135-9 Hence these 25-1 volumes yield—](https://iiif.wellcomecollection.org/image/b21449016_0718.jp2/full/800%2C/0/default.jpg)