A treatise on chemistry. Vol. 1, The non-metallic elements / by Sir H.E. Roscoe & C. Schorlemmer ...
- Henry Enfield Roscoe
- Date:
- 1881
Licence: Public Domain Mark
Credit: A treatise on chemistry. Vol. 1, The non-metallic elements / by Sir H.E. Roscoe & C. Schorlemmer ... Source: Wellcome Collection.
Provider: This material has been provided by University of Bristol Library. The original may be consulted at University of Bristol Library.
758/792 (page 742)
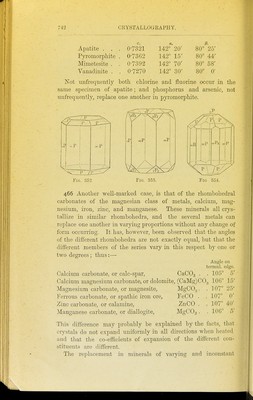
![Apatite . . Pyroniorphite Mimetesite . Vanadinite . 0-7321 0-7362 0-7392 0-7270 142° 20' 142 15' 142° 70' 142° 30' /3. 80° 25' 80° 44' 80° 58' 80° 0' Not iinfrequently both chlorine and fluorine occur in the same specimen of apatite; and phosphorus and arsenic, not unfrequently, replace one another in pyromorphite. >P2 Fig. 352 Fig. 353. Fig 354. 466 Another well-marked case, is that of the rhombohedral carbonates of the magnesian class of metals, calcium, mag- nesium, iron, zinc, and manganese. These minerals all crys- tallize in similar rhombohedra, and the several metals can replace one another in varying proportions -udthout any change of form occurring. It has, however, been observed that the angles of the different rhombohedra are not exactly equal, but that the different members of the series vary in this respect by one or two degrees; thus:— Calcium carbonate, or calc-spar, CaCO, Angle on termnl. edge. . 105° 5' Calcium magnesium carbonate, or dolomite, (CaMg)C03 106° 15' Magnesium carbonate, or magnesite, MgCOg. . 107° 25' Ferrous carbonate, or spathic iron ore, FeCO . . 107 0' Zinc carbonate, or calamine, ZnCO . . 107° 40' Manganese carbonate, or diallogite, MgCOs. . 106° 5' This difference may probably be explained by the facts, that crystals do not expand uniformly in all directions wlien heated and that the co-efficients of expansion of the different con- stituents are different. T]ie replacement in minerals of varying and inconstant](https://iiif.wellcomecollection.org/image/b21449016_0758.jp2/full/800%2C/0/default.jpg)