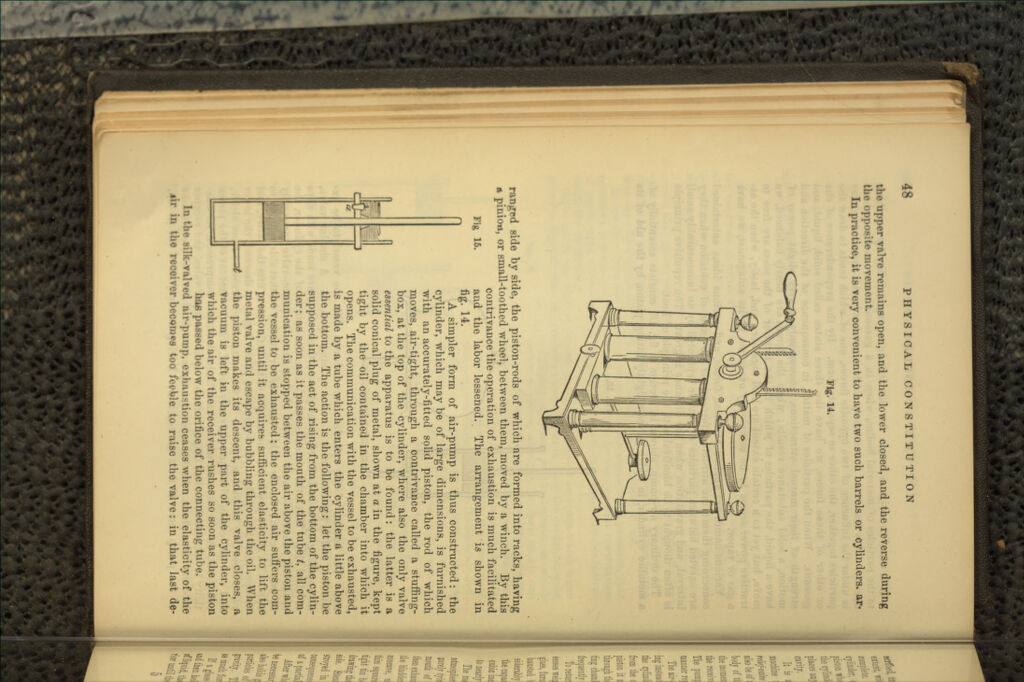
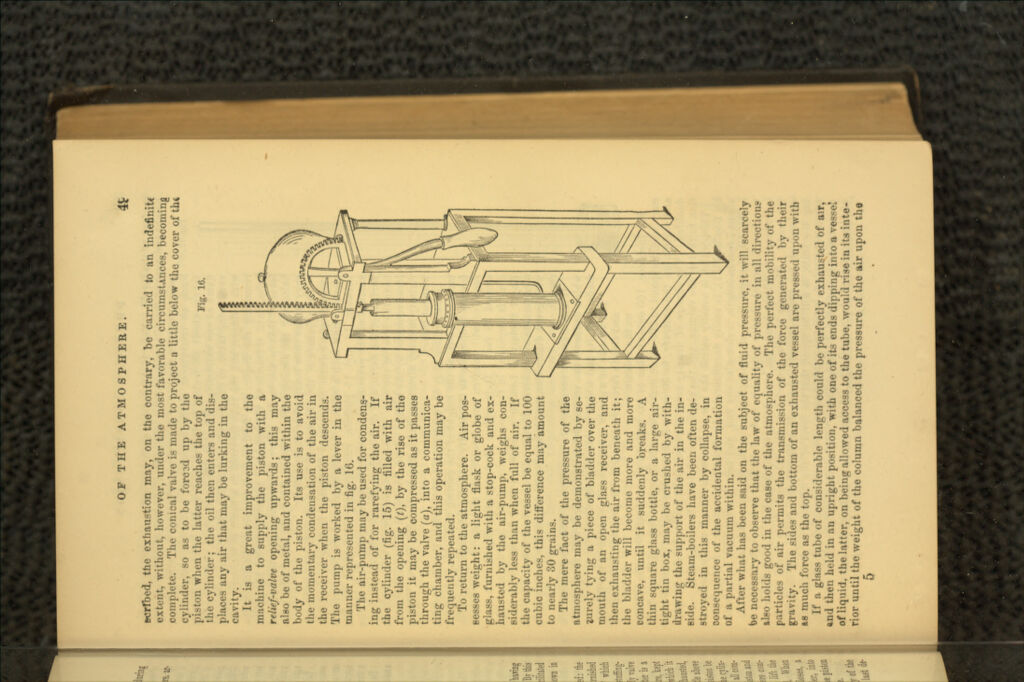
A manual of elementary chemistry : theoretical and practical / by George Fownes.
- George Fownes
- Date:
- 1868
Licence: Public Domain Mark
Credit: A manual of elementary chemistry : theoretical and practical / by George Fownes. Source: Wellcome Collection.
37/608 (page 45)
![fnto the hands of the physician;l it may be packed into a pocket case, with & little jar and a thermometer, and is always ready for use.3 The determination of the specific gravity of gases and vapors of volatile liquids is a problem of very great practical importance to the chemist: the theory of the operation is as simple as when liquids themselves are concerned, but the processes are much more delicate, and involve besides certain correc- tions for differences of temperature and pressure, founded on principles yet to be discussed. It will be proper to defer the consideration of these matters for the present. . The method of determining the specific gravity of a gas will be found described under the head of Oxygen, and that of the vapor of a volatile liquid in the Introduction to Organic Chemistry.8 1 This and other instruments described or figured in the course of the work, may be had of Murray :md Heath, 43 1'iccadilly, upon the excellence of whose workmanship reliance I ilKVd. a [The graduation of the urinometer la such that each degree represents 1-1000, thus giving the actual specific gravity without calculation, for the number of degrees on the scale cut by tin- surface of the liquid when this instrument is at rest, added to 1000 will represent the il.-iKity of the li.iui.l. If. for example, the surface of the liquid coincide with 23 on the scale, the specific gravity will be 1023, about the average density of healthy urine. — H. B.] [• The mode of determining the specific-gravity of a liquid by means of a solid Fig. 1L hns been omitted in the text. It results from the theorem of Archimedes, that if aiiv M-Ii'l !•«• immersed in water and then in any other liquid, the loss of weight sustained in each ca-e will give the relative weights of equal bulks of the liquids, and on dividing the weight of the liquid by the weight of the water, the quotient will IM? the specific-gravity of the liquid experimented on. For in- stance, let a piece of glass rod be suspended from the balance-pan and(exactly counterpoised, then immerse it in water and restore the equipoise by weights added to the pan to which the glass is suspended, the amount will give the loss of weight by immersion or the weight of a bulk of water equal to that of the \ >w wipe the glass dry, and having removed the additional weights, im- [| in the other liquid, and restore the equipoise as before; this latter wfiirht is the weight of a bulk of the liquid equal to that of the water. The Utter divided by the former gives the specific-gravity. For example: — The glass rod loses by immersion in water 171 grain*. The glass rod loses by immersion in alcohol... 143 143 ~ = -836 the specific-gravity required. - R. B^](https://iiif.wellcomecollection.org/image/b21183806_0037.jp2/full/800%2C/0/default.jpg)