Elements of physics for medical students / by Frederic James M. Page.
- Page, Frederic James M.
- Date:
- 1907
Licence: In copyright
Credit: Elements of physics for medical students / by Frederic James M. Page. Source: Wellcome Collection.
Provider: This material has been provided by Royal College of Physicians, London. The original may be consulted at Royal College of Physicians, London.
287/320 (page 265)
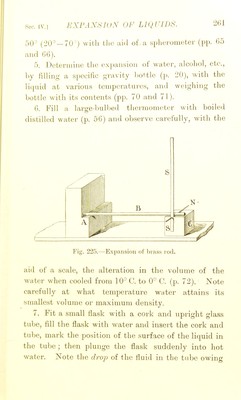
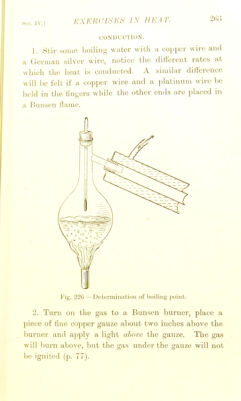
![S,K'. IV. 1 VAI'Oll K rUKSSUKK. Measure rlic v!ij)our pics.suie of water and of alcohol at various temperatures liy the apparatus shown on p. 1. Compare your results witli tliosc on p. LATKNT HEAT. 1. Mix 100 grams of water at 0' C. with 100 grains of water at 80^ and note the temperature after mixing. 2. Repeat the experiment, using 100 grams of ice at 0'^ instead of water, and note the resulting tem- perature. Calculate the latent heat of water (p. 88). 3. Fit up Mask, etc., as seen in Fig. 84, p. 96, but in addition tit a piece of straight glass tube to reach down into the liquid and long enough to stand 3 in. or 4 in. above the cork. Measure 100 c.c. of water into the flask b and 200 c.c. into the calorimeter. Boil the water until a rise of about 15° is observed in the water in the calorimeter; note the temperature accurately. When cold re-measure the water in a and B ; the loss gives the water converted into steam. Then calculate from your data the latent heat of steam. DEW-i^OINT. Determine this— (a) by stirring small ])ieces of ice into some water contained in a polished metal vessel until dew forms on the surface. Read off the temperature when this first takes place, then allow the vessel to stand till](https://iiif.wellcomecollection.org/image/b22651998_0287.jp2/full/800%2C/0/default.jpg)