A short manual of analytical chemistry : qualitative and quantitative organic and inorganic following the course of instruction given in the laboratories of the South London School of Pharmacy / by John Muter.
- Muter, John, 1841-1912.
- Date:
- 1903
Licence: In copyright
Credit: A short manual of analytical chemistry : qualitative and quantitative organic and inorganic following the course of instruction given in the laboratories of the South London School of Pharmacy / by John Muter. Source: Wellcome Collection.
80/264 (page 60)
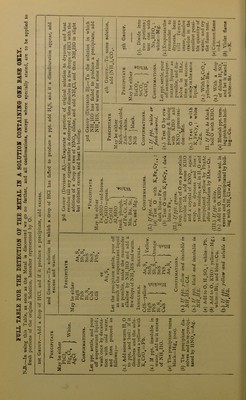
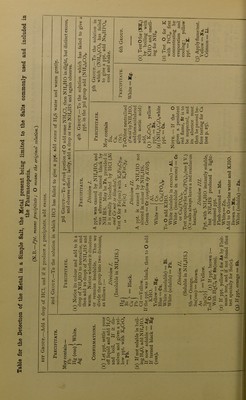
![QUALITATIVE ANALYSIS. be suspected. In the case of a complex solution in which a salt of some other metal is given dissolved in excess of an alkali, an intimation of the fact will be obtained on cautiously adding the HC1, as, at the moment of neu- tralisation, the dissolved substance appears as a precipitate before again dissolv- ing in the excess of HC1. Basic plumbic acetate has an alkaline reaction. Step 2. Dip a clean platinum wire in the solution, or, if a solid, moisten the wire with HC1, dip it in the powdered substance, and heat in the inner Bunsen or blowpipe flame. The outer flame is coloured as under :— Violet Golden-yellow Yellowish-green Crimson Orange-red Green Blue . Potassium. Sodium. Barium. Strontium or Lithium. Calcium. Copper or Boracic acid. Lead, Arsenic, Bismuth ; also Copper as chloride. Step 3. Heat a little of the solid substance (or the residue left on evapora- tion if in solution) on charcoal before the blowpipe. Ordinary alkaline salts fuse and sink into the charcoal; some decre- pitating (example NaCl, etc.), others deflagrating (as KN03, KC103, etc.), but no sufficiently characteristic indications are usually obtained, except in one of the following cases :— A. A white luminous residue is left. Moisten it when cold with a drop or two of cobaltous nitrate, and again apply the blowpipe, observing any change of colour as follows :— The residue becomes blue, indicating Al, Silicates, Phosphates, or Borates. >> >> >) green, ,, Zn. ,, „ „ pink or flesh-coloured, indicating Mg. B. A coloured residue is left. Prepare a borax bead, and heat a little of the substance in it, both in the reducing and oxidising flame, and note any colours corresponding with the following list:— Metal. In Oxidising Flame. In Reducing Flame. Cu Green (hot). Blue (cold). Red (cold). Co Blue. Blue. Cr Green. Green. Fe Red (hot). Yellowish (cold). Bottle-green. Mn Amethyst. Colourless. Ni Reddish-brown (hot). Yellow (cold). Same as oxidising flame. C. A metallic residue is left, with or without incrustation surrounding it. Mix a little of the substance with KCN and NaXOs, and expose on charcoal to the reducing flame. (a) Metallic globules are produced without any surrounding incrusta- tion of oxide. This occurs with Ag, Au, Cu, Fe, Co, and Ni, all easily recognisable. (b) Metallic globules are produced with a surrounding incrustation of oxide. This occurs with Sn, Bi, Pb, and Sb; the incrustation having the characteristic colours already described in Case I., Step i, G. Note.^-Sb often forms a white and distinctly crystalline crust. (c) The metal volatilises, and only leaves an incrustation of oxide. This occurs with As (odour of garlic, and white incrustation), Zn (yellow [hot], white [cold]), and Cd (reddish-brown).](https://iiif.wellcomecollection.org/image/b28137590_0080.jp2/full/800%2C/0/default.jpg)