The solubilities of the pharmacopœial organic acids and their salts / by Atherton Seidell.
- Seidell, Atherton, 1878-1961.
- Date:
- [1910]
Licence: In copyright
Credit: The solubilities of the pharmacopœial organic acids and their salts / by Atherton Seidell. Source: Wellcome Collection.
14/104 (page 12)
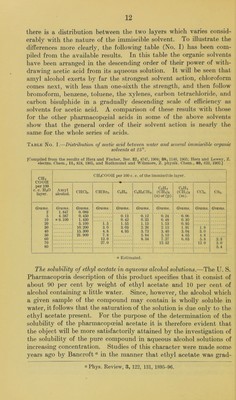
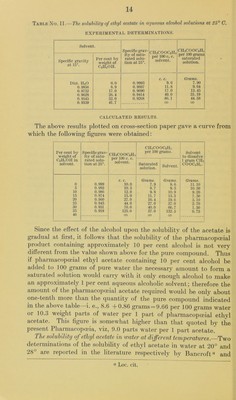
![there is a distribution between the two layers which varies consid- erably with the nature of the immiscible solvent. To illustrate the differences more clearly, the following table (No. I) has been com- piled from the available results. In this table the organic solvents have been arranged in the descending order of their power of with- drawing acetic acid from its aqueous solution. It will be seen that amyl alcohol exerts by far the strongest solvent action, chloroform comes next, with less than one-sixth the strength, and then follow bromoform, benzene, toluene, the xylenes, carbon tetrachloride, and carbon bisulphide in a gradually descending scale of efficiency as solvents for acetic acid. A comparison of these results with those for the other pharmacopoeial acids in some of the above solvents show that the general order of their solvent action is nearl}’’ the same for the whole series of acids. Table No. I.—Distribution of acetic acid between water and several immiscible organic solvents at 25°. [Compiled from the results of Herz and Fischer, Ber. 87, 4747, 1904; 88, 1140, 190.5; Herz and Lewey, Z. electro. Chem., 11, 818, 1905, and Rothmund and Wilsmore, Z. physik. Chem., 40, 623, 1902.] CHs COOH per 100 c. c. HjO layer. CH3COOH per 100 c. c. of the immiscible layer. Amyl alcohol. CHCI3. CHBr3. CeHs. CeHsCHa. CeH^ (CHs)2 (o)or (p). CeH, (CH3)2 (m). ecu. CSj. Grams. 2 5 10 20 30 40 50 60 70 80 Grams. 1.847 4.587 a 9.100 Grams. 0.089 0.450 1.430 5.100 10.200 15.300 21.900 Grams. Grams. Grams. Grams. Grams. Grams. Grams. i.5 3.0 4.8 7.8 12.0 27.0 0.13 0.42 1.55 3.03 4.95 0.12 0.33 1.13 2.26 3.73 5. 84 8. 34 0.24 0. 48 1.13 2.15 3.40 5.10 7.27 12.52 0.06 0.30 0.95 1.91 3.04 4.65 6.65 1.8 3.0 4.8 6.8 12.0 2.3 3.0 5.4 o Estimated. The solubility of ethyl acetate in aqueous alcohol solutions.—The U. S. Pharmacopoeia description of this product specifies that it consist of about 90 per cent by weight of ethyl acetate and 10 per cent of alcohol containing aTittle water. Since, however, the alcohol which a given sample of the compound may contain is wholly soluble in water, it follows that the saturation of the solution is due only to the ethyl acetate present. For the purpose of the determination of the solubility of the pharmacopoeial acetate it is therefore evident that the object will be more satisfactorily attained by the investigation of the solubility of the pure compound in aqueous alcohol solutions of increasing concentration. Studies of this character were made some years ago by Bancroft “ in the manner that ethyl acetate was grad- « Phys. Review, 3, 122, 131, 1895-96.](https://iiif.wellcomecollection.org/image/b28063909_0014.jp2/full/800%2C/0/default.jpg)