The solubilities of the pharmacopœial organic acids and their salts / by Atherton Seidell.
- Seidell, Atherton, 1878-1961.
- Date:
- [1910]
Licence: In copyright
Credit: The solubilities of the pharmacopœial organic acids and their salts / by Atherton Seidell. Source: Wellcome Collection.
55/104 (page 53)
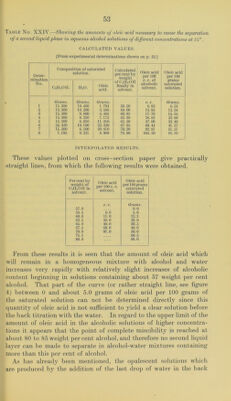
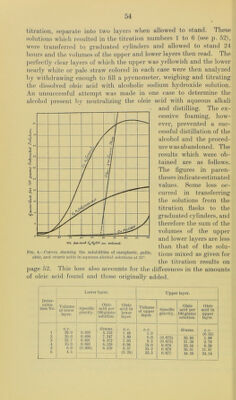
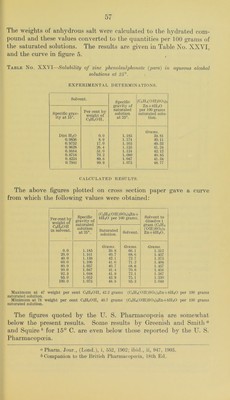
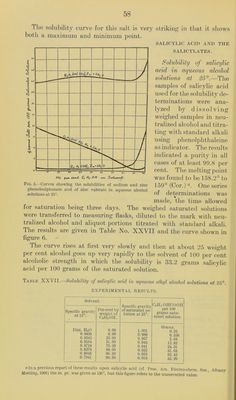
![Table No. XXIV.—Shoiving the amounts of oleic acid necessary to cause the separation of a second liquid phase in aqueous alcohol solutions of different concentrations at 25°. CALCULATED VALUES. [From experimental determinations shown on p. 52.] Deter- mination No. Composition of saturated solution. Calculated per cent by weight of C2H5OH finally In solvent. Oleic acid per 100 c. c. of alcoholic solvent. Oleic acid per 100 grams saturated solution. C2lI.'iOII. HjO. Oleic acid. Orams. Orams. Grams. c. c. Orams. 1 15.300 10. 400 1.794 59. 50 6.92 6.53 2 15. 300 10. 200 3.588 60.00 13.93 12.34 3 15. 300 9.800 4. 485 60.95 17.70 15.16 4 15.300 9. 250 7.175 62.30 28.83 22.60 5 15.300 8.050 11.210 65.50 47.09 32. 40 6 24. 420 10.100 22. 420 67.95 69. 44 41.57 7 15. 300 6. 500 20. 810 70. 20 92.81 51.57 8 1.195 0. 321 8.969 78.80 645.20 85.10 INTERPOLATED RESULTS. These values plotted on cross-section paper give practical!}^ straight lines, from which the following results were obtained. Per cent by weight of C2H5OH in solvent. Oleic acid per 100 c. c. solvent. Oleic acid per 100 grams saturated solution. 57.0 c. c. Orams. 0.0 58.5 6.6 5.0 60.0 11.0 12.3 62.5 30.0 20.0 65.0 49.0 30.5 67.5 69.0 40.0 70.0 91.0 50.0 75.5 68.5 80.0 — 88.0 From these results it is seen that the amount of oleic acid which will remain in a homogeneous mixture with alcohol and water increases very rapidly with relatively slight increases of alcoholic content beginning in solutions containing about 57 weight per cent alcohol. That part of the curve (or rather straight line, see figure 4) between 0 and about 5.0 grams of oleic acid per 100 grams of the saturated solution can not be determined directly since this quantity of oleic acid is not sufficient to yield a clear solution before the back titration with the water. In regard to the upper limit of the amount of oleic acid in the alcoholic solutions of higher concentra- tions it appears that the point of complete miscibility is reached at about 80 to 85 weight percent alcohol, and therefore no second liquid layer can be made to separate in alcohol-water mixtures containing more than this per cent of alcohol. As has already been mentioned, the opalescent solutions which are produced by the addition of the last drop of water in the back](https://iiif.wellcomecollection.org/image/b28063909_0055.jp2/full/800%2C/0/default.jpg)