The solubilities of the pharmacopœial organic acids and their salts / by Atherton Seidell.
- Seidell, Atherton, 1878-1961.
- Date:
- [1910]
Licence: In copyright
Credit: The solubilities of the pharmacopœial organic acids and their salts / by Atherton Seidell. Source: Wellcome Collection.
64/104 (page 62)
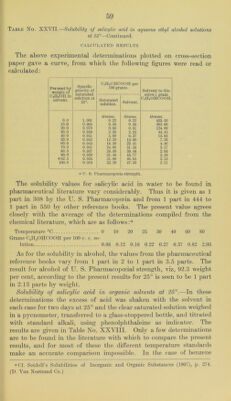
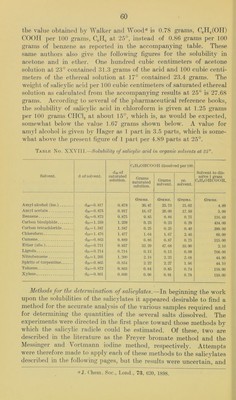
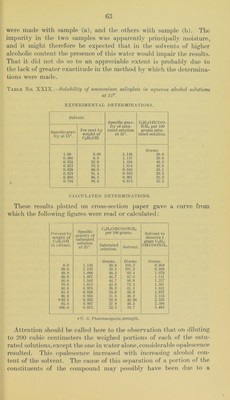
![diluted to 500 cubic centimeters. An excess of IICl was added to aliquot portions of this solution, which were then eva[)orated to dry- ness on the steam bath and either filtered and just neutralized with alkali before the titration with standard silver nitrate, or the residue (determination No. 2) was gently ignited to remove the free salicylic acid before the titration for chlorides. Determi- nation No. Salicylate solution. CeinOIICOONa. 0.1 N ArNOs required. Calc. CsinOHCOONa. C. c. Grams. C. c. Per cent. 1 25 0.250 15. 0 99.9 2 50 0.500 31.3 100.2 3 75 0.750 47.0 100. 3 These results show that in the case of sodium salicylate this method is as reliable as that of the pharmacopoeia; that it will prove equally so in the case of the other salicylates is hardly to be doubted. If further experiments should confirm this expectation we would then have a single procedure for these salts instead of three as is now the case. Solubility of ammonium salicylate in aqueous alcohol solutions.—In the case of this compound two series of determinations were made with two samples of the salt. In one case the solutions were allowed two days for reaching equilibrium and in the other something over a week. It was evident that the shorter time was am])le; in fact.it seems probable that even ten hours would have been sufficient. The two samples of material were each analyzed as follows: A weighed quantity of the salt was transferred to a 200 cubic centimeter flask, dissolved in water and diluted to the mark. Aliquot })ortions of the solution containing 1 to 2 grams ammonium salicylate v^ere j)laced in a Kjeldahl distilling flask with 25 cubic centimeters of normal alkali. The liberated ammonia was distilled into 25 cubic centi- meters of normal acid, and the excess of acid in the receiver titrated back with standard alkali and the excess of alkali in the distil- lation flask titrated back with standard acid. The amount of alkali equivalent to the ammonia in both cases agreed fairly satisfactorily. From these readings the amount of ammonia and thus of the am- monium salicylate in the aliquot portion of the solution was easily calculated. According to this method the two samples gave the fol- lowing results expressed in the percentage of Ccir40II.C00NH4 present. Sample (a), 96.8 per cent; sample (b), 98.7 per cent. Although one of these samples was below the United States Pharma- copoeia requirement and the other above, the solubility determina- tions made with them gave a curve in which no irregularities could be traced definitely to either sample. It may be mentioned that of the results shown in Table No. XXIX, the second, third, and sixth](https://iiif.wellcomecollection.org/image/b28063909_0064.jp2/full/800%2C/0/default.jpg)