The solubilities of the pharmacopœial organic acids and their salts / by Atherton Seidell.
- Seidell, Atherton, 1878-1961.
- Date:
- [1910]
Licence: In copyright
Credit: The solubilities of the pharmacopœial organic acids and their salts / by Atherton Seidell. Source: Wellcome Collection.
76/104 (page 74)
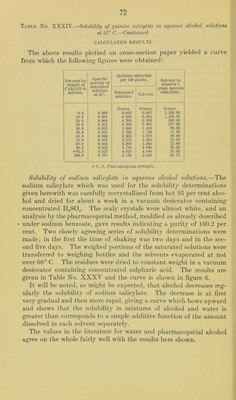
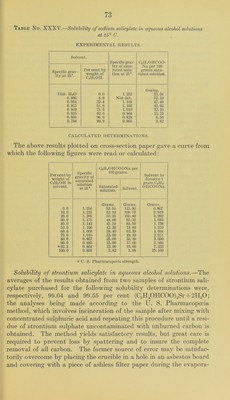
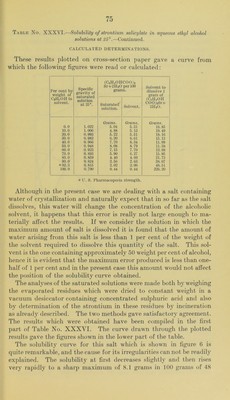
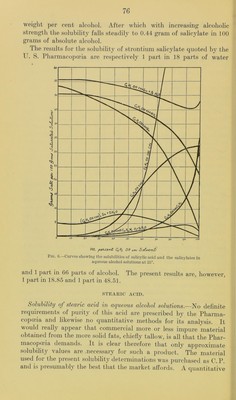
![tion of the excess of sul])liuric acid, the filter paper being allowed to drop into the crucible when the teni])erature is raised to redness. The better of the above-mentioned samples of strontium salicylate was reciystallized from hot 80 to 90 })er cent alcohol and the crystals dried in a vacuum desiccator over concentrated sulphuric acid for about a week. The product analyzed practically 100 per cent (CJI^0IIC00)2Sr + 2H20, showing that no loss of water of crystal- lization had occurred in drying the material as described. The tubes for the solubility determinations were prepared as has already been described, but after the period of rotation it was noticed that the solid phase in the tube containing the solvent of 99.9 per cent alcoholic strength had been converted into an amorphous bulky white powder. It therefore appeared that absolute alcohol is able to remove some or all of the water of crystallization of strontium salicylate. This point was tested by preparing a quantity of the dehydrated salt by allowing some of the crystallized material to stand in contact with absolute alcohol until it had practically all been converted to the powder form and then filtering, washing, and drying. Solubility determinations made with this product did not differ appreciably from those obtained for the crystalline dihydrate. Analyses of it gave an amount of strontium sulphate which corre- sponded to 93.87 percent (C6ll40HC00)2Sr, 99.49 per cent of mono- hydrate or 100.85 per cent of (C6H40IIC00)2Sr-|-li^H20. These results show that although absolute alcohol changes the appearance of the ordinary strontium salicylate very materially the amount of dehydration which it effects is comparatively small. Under the high power microscope the salicylate powder shows minute irregular crystals and no appreciable amount of amorphous material. Four series of solubility determinations were made, in three of which the crystalline salicylate was used, and in the other the powder obtained by treating the crystals with absolute alcohol. The results in all cases were in satisfactory agreement. Table No. XXXVI.—Solubility of strontium salicylate in aqueous ethyl alcohol solutions at 25°. EXPERIMENTAL DETERMINATIONS. Solvent. Specific grav- ity of satu- rated solu- tion at 25°. (C6H40HC00)2 Sr-|-2H20 per 100 grams satu- rated solution. Specific grav- ity at 15°. Per cent by weight of CjHoOH. Dist. IIjO 0.0 1.022 Grams. 5.04 0.980 8.9 1.007 4.91 0.954 32.4 0.979 0.55 0.915 51.0 0.945 8.02 0.809 71.5 0.891 5.80 0. 804 90. 0 0.804 1.24 0. 794 99.9 0.790 0.44](https://iiif.wellcomecollection.org/image/b28063909_0076.jp2/full/800%2C/0/default.jpg)