The solubilities of the pharmacopœial organic acids and their salts / by Atherton Seidell.
- Seidell, Atherton, 1878-1961.
- Date:
- [1910]
Licence: In copyright
Credit: The solubilities of the pharmacopœial organic acids and their salts / by Atherton Seidell. Source: Wellcome Collection.
81/104 (page 79)
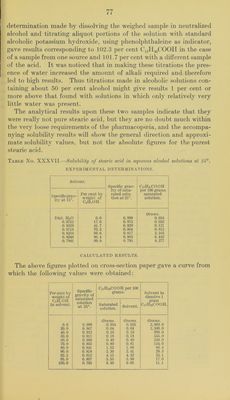
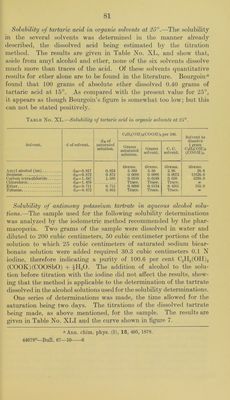
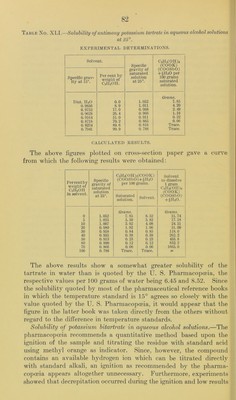
![TANNIC ACID. Attempts to determine the solubility in aqueous alcohol solutions of a sample of tannic acid “ of apparently very good quality were unsuccessful on account of the impossibility of saturating the solu- tion with the acid. So much of the sample dissolved in water and also in absolute alcohol that before an excess of it remained undis- solved the solution had become so viscous that it flowed from one end of the tube to the other only with extreme slowness. It appeared doubtful whether it would have been possible to separate the satu- rated solution from the undissolved material even if the saturation point could have been reached. Furthermore, there was apparently no satisfactory method available for analyzing the saturated solution even if it could have been obtained. It was therefore necessary to abandon attempts to determine the solubility of this acid in the manner followed for the remaining members of the series of pharma- copoeia! compounds. According to the literature the solubility in water is given all the way from 1 part in 0.35 parts of water to 1 part in 5.0 parts, and the solubility in alcohol from 1 part in 0.23 to 1 part in 2.0. The state- ment that it is almost insoluble in absolute alcohol occurring in sev- eral reference books is, according to the experiment mentioned above, certainly open to question. TARTARIC ACID AND THE TARTRATES. Solubility of tartaric acid in aqueous alcohol solutions.—The sample useil for the solubility determination was analyzed by titration with normal alkali, using phenol])hthaleine as indicator. The results cor- responded to a purity of 99.3 per cent C2ll2(OII)2(COOH)2. The melting point was found to be 166.1° to 167.3° (cor.). One series of determinations was made and the time allowed for saturation, with constant shaking, was two days. The saturated solu- tions were weighed and, after dilution, aliquot portions were titrated with normal alkali. The results are given in Table No. XXXIX and the curve shown in figure 7. The decrease in solubility with increase of alcoholic content of the solution is perfectly regular, but at no point is the sum of the amounts which the alcohol and water in each solvent would dissolve independently as great as actually found. The figure for water agrees fairly well with that quoted by the pharmacopoeia, but with alcohol of IT. S. Pharmacopoeia strength the difference is much greater. A series of determinations of the solubility of tartaric acid in water at olifferent temperatures publisheol by Leidie ^ contains a value for a Kindly furnished by Dr. Geo. W. Hoover, of the Drug Laboratory, Bureau of Chemistry, U. S. Department of Agriculture. b Compt. rend., 95, 87, 1882.](https://iiif.wellcomecollection.org/image/b28063909_0081.jp2/full/800%2C/0/default.jpg)