The solubilities of the pharmacopœial organic acids and their salts / by Atherton Seidell.
- Seidell, Atherton, 1878-1961.
- Date:
- [1910]
Licence: In copyright
Credit: The solubilities of the pharmacopœial organic acids and their salts / by Atherton Seidell. Source: Wellcome Collection.
85/104 (page 83)
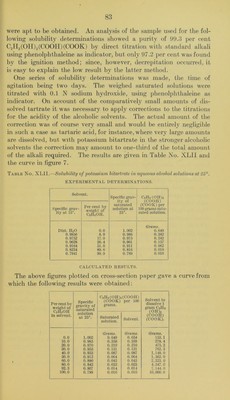
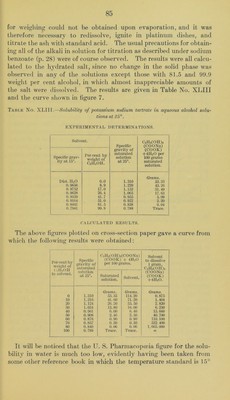
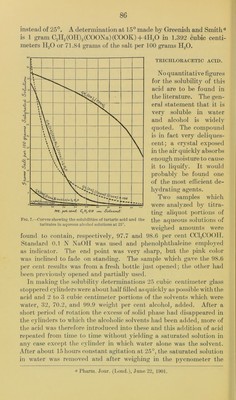
![were apt to be obtained. An analysis of the sani])le used for the fol- lowing solubility determinations showed a purity of 99.3 per cent C2H2(0II)2(C00II)(C00K) by direct titration witli standard alkali using phenolphthaleine as indicator, but only 97.2 per cent was found by the ignition method; since, however, decrepitation occurred, it is easy to explain the low result by the latter method. One series of solubility determinations was made, the time of agitation being two days. The weighed saturated solutions were titrated with 0.1 N sodium hydroxide, using phenolphthaleine as indicator. On account of the comparatively small amounts of dis- solved tartrate it was necessary to apply corrections to the titrations for the acidity of the alcoholic solvents. The actual amount of the correction was of course very small and would be entirely negligible in such a case as tartaric acid, for instance, where veiy large amounts are dissolved, but with potassium bitartrate in the stronger alcoholic solvents the correction may amount to one-third of the total amount of the alkali required. The results are given in Table No. XLIl and the curve in figure 7. Table No. XLII.—Solubility of potassiuin bitartrate in aqueous alcohol solutions at 25°. EXPERIMENTAL DETERMINATIONS. Solvent. Specific grav- ity of saturated solution at 25°. C2H2 (011)2 (COOll) (COOK) per 100 grams satu- rated solution. Specific grav- ity at 15°. Per cent by weight of C2H5OH. Dist. HjO 0.0 1.002 Grams. 0.649 0.9856 8.9 0.986 0.382 0.9752 17.0 0.975 0.242 0.9628 26.4 0.961 0.157 0.9164 51.0 0.911 0.062 0.8234 89.6 0.816 0.018 0.7941 99.9 0.789 0.010 CALCULATED RESULTS. The above figures plotted on cross-section paper gave a curve from which the following results were obtained: Per cent by weight of C2H5OH in solvent. Specific gravity of saturated C2H2(0H)2(C00H) (COOK) per 100 grams. Solvent to dissolve 1 gram C2H2 (0H)2 (COOH) (COOK). solution at 25°. Saturated solution. Solvent. 0.0 1.002 Grams. 0. 649 Grams. 0.654 Grams. 153.1 10.0 0.985 0.358 0.359 278.4 20.0 0.970 0.210 0.210 475.2 30.0 0.953 0.131 0.131 762.3 40.0 0.933 0.087 0.087 1.148.0 50.0 0.912 0.064 0.064 1,562.0 60.0 0.890 0.043 0.043 2,323. 0 80.0 0.842 0.023 0.023 4,347.0 92.3 0.807 0.014 0.014 7,144.0 100.0 0.789 0.010 0.010 10,000.0](https://iiif.wellcomecollection.org/image/b28063909_0085.jp2/full/800%2C/0/default.jpg)