The solubilities of the pharmacopœial organic acids and their salts / by Atherton Seidell.
- Seidell, Atherton, 1878-1961.
- Date:
- [1910]
Licence: In copyright
Credit: The solubilities of the pharmacopœial organic acids and their salts / by Atherton Seidell. Source: Wellcome Collection.
89/104 (page 87)
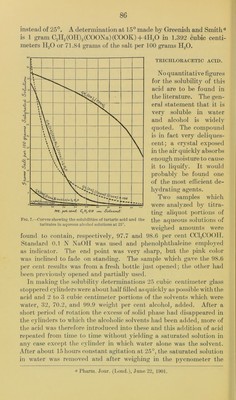
![amountof the trichloracetic acid deteriiiined by titration. The follow- ing results were obtained; Solubility of trichloracetic acid in water at 25°. Specific gravity of saturated solution at 25° 1. 615 Grams CfUjCOOH per 100 grams saturated solution 92. 32 Grams CCI3COOH per 100 grams water 1, 201. 00 Grams H2O to dissolve 1 gram CCI3COOII 0. 0832 Since my supply of trichloracetic acid was nearly exhausted before either of the alcoholic solutions was saturated at 25°, I lowered the temperature of the bath to 15° and hoped that an additional crystal of trichloracetic acid in each cylinder would cause the separation of the excess of acid which might possibly be present at the lower tem- perature. Tlie added crystals dissolved, however, as quickly as before and there was no further ])ossibilitv of obtaining saturated solutions. Even the aqueous solution to which a little more water had been added contained none of the undissolved acid. Althou<rh none of the four solutions were saturated at 15°, I thouglit it would nevertheless be of interest to determine the quantity of acid present in each and thus have figures which would show that the actual solu- bility was not below the amounts found. In the case of the aqueous solution the following residts were obtained: Specific gravity of solution (not quite saturated) at 15° 1. 593 Grams Cri3COOII per 100 grams of the solution 87. 22 Thus it is certain that the solubility of trichloracetic acid in water at 15° is not less than 87.22 grams per 100 grams of the saturated solution. Now, in the case of the solution made with trichloracetic acid and alcohol of 99.9 weight per cent the specific gravity was found to be 1.508. On diluting 10 cubic centimeters of this solution with water in order to determine the dissolved acid by titration of an aliquot part, a second liquid layer amounting to about 4 cubic centimeters separated. The aqueous part having been diluted to 500 cubic centimeters was found by titration to contain 8.9475 grams CCI3COOH. It gave no opalescence with silver nitrate, showing that by whatever reaction the second compound had been formed no free hydrochloric acid had been liberated. It appeared most probable that the ethyl ester of the trichloracetic acid had been formed, no doubt under the influ- ence of the very great dehydrating power of the acid. That the second liquid phase was really the trichloracetic acid ester was proved by separating and determining its boiling point which was found to be 162° to 165°. The boiling point as given by Beilstein is 164°. The lic^uid obtained therefore by attempting to prepare a saturated solution of trichloracetic acid in absolute alcohol was really a mixture of the trichloracetic acid ethyl ester and trichlor-](https://iiif.wellcomecollection.org/image/b28063909_0089.jp2/full/800%2C/0/default.jpg)