The solubilities of the pharmacopœial organic acids and their salts / by Atherton Seidell.
- Seidell, Atherton, 1878-1961.
- Date:
- [1910]
Licence: In copyright
Credit: The solubilities of the pharmacopœial organic acids and their salts / by Atherton Seidell. Source: Wellcome Collection.
90/104 (page 88)
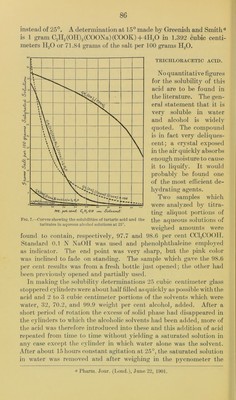
![acetic acid containing a little water. In adding water to this mixture a separation occurred at a certain })oint and it therefore appeared of interest to determine the amount of water necessary to just cause this separation and also the amount required to dissolve all of the acid out of the ester solution. The experiment was made as follows: Ten cubic centimeters of the solution was transferred to a graduated cylinder and water added slowly from a burette. It was found that 5.9 cubic centimeters of 11,0 just produced a faint opalescence at 15°, 6.05 cubic centimeters gave a milky solution which showed no tendency to separate into two layers until 7.1 cubic centimeters of H2O had been added and then on standing the two layers meas- ured respectively 9.2 cubic centimeters for the lower and 7.6 cubic centimeters for the u])per. Now, by adding more water and shaking, the volume of the lower layer gradually diminished, so that with a total of 8.0 cubic centimeters of water the lower layer measured 8.3 cubic centimeters and with 10 cubic centimeters of water it became 7.2 cubic centimeters in volume. At this point an aliquot portion of the upper layer was removed and on titration it was found that 5.104 grams of CCI3COOII were present in the whole of the upper layer. According to the previous analysis (p. '87) in which an equal volume of the solution had been diluted to 500 cubic centimeters the amount of acid found was 8.9475 grams, therefore in adding 10 cubic centimeters instead of 500 cubic centi- meters of water the removal of the trichloracetic acid from the ester was not complete. In order to ascertain how much additional water would be necessary to dissolve out the remaining (8.9475 — 5.104 = ) 3.8435 grams of acid, the upper layer was siphoned off as completely as possible from the 7.1 cubic centimeters of lower layer and successive amounts of water were added and the mixture shaken after each addition. It was found that with 3 cubic centi- meters of H2O the lower layer was reduced in volume to 5.4 cubic centimeters, with 8 cubic centimeters it became 4.6 cubic centimeters, and with 18 cubic centimeters it had become 4.0 cubic centimeters. The aqueous layer was then found to contain a total of 3.226 grams of (X'lgCOOII, or nearly the calculate amount which should have been recovered. The two solutions which had been prepared with 70.2 and 32 weight per cent alcohol respectively were also titrated with water as just described for the solution in which 99.9 weight per cent alcohol had been used. The residts were as follows: Five cubic centimeters of the first (the 70.2 per cent alcoholic solution, required 7.3 cubic centimeters of II2O to produce opales- cence; when 9.0 cubic centimeters of II2O had been added the lay- ers separated within about one-half hour into 1.4 cubic centimeters of lower and 12.1 cubic centimeters of upper layer. With 15 cubic *](https://iiif.wellcomecollection.org/image/b28063909_0090.jp2/full/800%2C/0/default.jpg)