The solubilities of the pharmacopœial organic acids and their salts / by Atherton Seidell.
- Seidell, Atherton, 1878-1961.
- Date:
- [1910]
Licence: In copyright
Credit: The solubilities of the pharmacopœial organic acids and their salts / by Atherton Seidell. Source: Wellcome Collection.
91/104 (page 89)
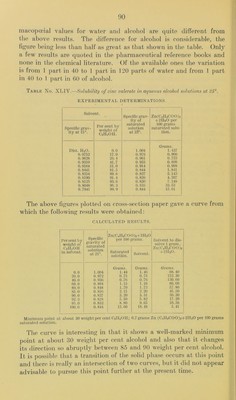
![centimeters of II2O the volume of the lower layer had been reduced to 1.3 cubic centimeters and with 25 cubic centimeters of water it became 1.1 cubic centimeters. Five cubic centimeters of the second (the 32 per cent alcoholic solution) required 11.3 cubic centimeters of HoO to become opalescent, and when a total of 20 cubic centimeters H2O had been added the lower layer measured only about 0.5 cubic centimeter. These results show that the solubility of trichloracetic acid can not be determined in solutions containing any appreciable amount of alcohol since the ester of the f),cid will be formed and therefore alter the equilibrium of the system. It would no doubt be of much interest to determine the limits within which the ester may form and also the extent to which the alcohol is consumed in the redaction but such experiments are hardly within the scope of the present bulletin. VALERATES. The solubility of ammonium, valerate.—No requirements are made for this salt by the pharmacopoeia other than it be kept in well- stoppered bottles. A sample purchased for solubility determina- tions was in the form of almost colorless, apparently hygroscopic crystals which when analyzed by distillation of the ammonia gave results indicating a composition of CJIs,COONIl4-20^1900011. At- tempts to saturate a series of alcoholic solvents with this salt were unsuccessful. Since no particular composition is required by the pharmacopoeia for this compound it appeared useless to pre])are samples and make solubility determinations until some definite requirements are made for this salt. Solubility of zinc valerate in aqueous alcohol solutions.—No method is prescribed by the pharmacopoeia for the analysis of this salt, the determination of the zinc was therefore made as follows. An ali- quot portion of the solution of the weighed sample was heated to the boiling point and sodium carbonate solution added until the precipitation of the zinc as carbonate was complete. After diges- tion on the steam bath for about an hour the precipitate was filtered on a Gooch crucible, ignited, and weighed <as zinc oxide; the results indicated a purity of 97.8 per cent Zn (C4TT„C()0)2 + 2TI2O. Attempts to ignite the sample directly and determine the zinc by weighing the ignited residue gave low results, indicating that loss by volatilization had occurred. Two series of solubility determinations were made, the period of constant agitation being three da3^s in each case. The saturated .solutions were analyzed by precij)itation of the zinc as carbonate as above mentioned, and weighing as zinc oxide. The results are given in Table No. XLIV and the curve shown in figure 5. The phar-](https://iiif.wellcomecollection.org/image/b28063909_0091.jp2/full/800%2C/0/default.jpg)