Lessons in elementary chemistry : inorganic and organic / by Henry E. Roscoe.
- Date:
- 1869
Licence: Public Domain Mark
Credit: Lessons in elementary chemistry : inorganic and organic / by Henry E. Roscoe. Source: Wellcome Collection.
Provider: This material has been provided by the Royal College of Physicians of Edinburgh. The original may be consulted at the Royal College of Physicians of Edinburgh.
45/502 (page 31)
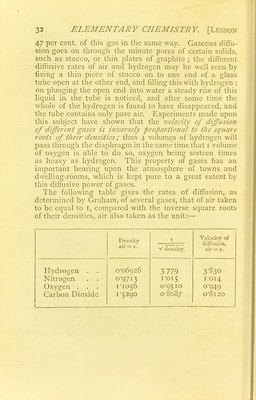
![of 10 litres, the temperature of the room being 150 C. and the barometer standing at 752 mm. We know (1) that 122-6 parts by weight of potassium chlorate yield 48 of oxygen ; (2) that a litre of oxygen at o° C. and 760 mm. weighs 1 4298 grams. We must now ask, What will 10 litres of oxygen weigh if measured at 150 C. and under the pressure of 752 mm. ? Now, 10 litres at o° j ^ -n i 10 X 760 X (273 + 15) T„.££, and 760mm. wdl become v J —— = io-66i 752x273 at 150 and 752mm.; therefore, if 10 litres at o° and 760mm. weigh I4298grams, iolitres at 150 and 752mm. will weigh 14 298 _ j r/j j grams. Next we require to know how ro66i & many grams of chlorate will furnish this weight of oxygen; as every 122*6 parts of chlorate yield 48 parts f . „ , i22-6 X I3'4i 1 r of oxvgen, we shall need —^-^— = 34 254 grams of 48 chlorate. In the same way we can calculate, for instance, the weight of zinc and sulphuric acid needed to inflate a balloon of the capacity of 150 cubic metres with hydrogen when the thermometer stands at n° C. and the barometer at 763 mm. [The student will do well to work out numerous examples of this kind, in order to familiarize himself with these methods of calculation (see Exercises at the end of the book).] Diffusion of Gases. Another physical property of gases is that of diffusion. Gases which, when mixed together, do not combine chemically, have the power of becoming intimately mixed together, even when differing in specific gravity, and when the heavier gas is placed at the bottom, and both remain at rest. This important property is called the diffusive pozvtr of gases. The rate at which gases diffuse varies greatly. Thus, a bottle filled with hydrogen lost 94^5 per cent, of this gas when left exposed to the air in the same time as that in which a bottle of carbonic acid lost only](https://iiif.wellcomecollection.org/image/b21927984_0045.jp2/full/800%2C/0/default.jpg)