Investigations on the purification of Boston sewage made at the sanitary research laboratory and sewage experiment station of the Massachusetts institute of technology, with a history of the sewage-disposal problem / By C.E.A. Winslow and Earle B. Phelps.
- Charles-Edward Amory Winslow
- Date:
- 1906
Licence: In copyright
Credit: Investigations on the purification of Boston sewage made at the sanitary research laboratory and sewage experiment station of the Massachusetts institute of technology, with a history of the sewage-disposal problem / By C.E.A. Winslow and Earle B. Phelps. Source: Wellcome Collection.
Provider: This material has been provided by London School of Hygiene & Tropical Medicine Library & Archives Service. The original may be consulted at London School of Hygiene & Tropical Medicine Library & Archives Service.
54/176 (page 48)
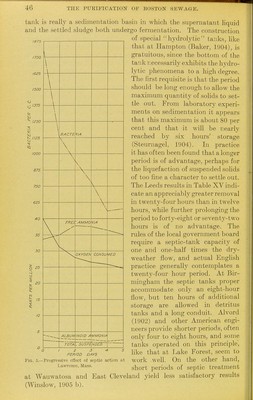
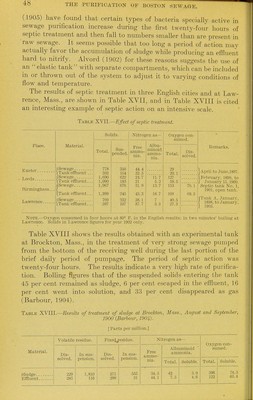
![(1905) have found that certain types of bacteria speciality active in sewage purification increase during the first twenty-four hours of septic treatment and then fall to numbers smaller than are present in raw sewage. It seems possible that too long a period of action may actually favor the accumulation of sludge while producing an effluent hard to nitrify. Alvord (1902) for these reasons suggests the use of an elastic tank with separate compartments, which can be included in or thrown out of the system to adjust it to varying conditions of flow and temperature. The results of septic treatment in three English cities and at Law- rence, Mass., are shown in Table XVII, and in Table XVIII is cited an interesting example of septic action on an intensive scale. Table XVII.—Eifect of septic treatment. Place. Exeter Leeds Birminglinm Lawrence Material. I Sewage ITank effluent /Sewage tXanlc effluent 'Sewage Tank effluent /Sewage ITanlc effluent Solids. Total. 778 593 1,690 1,090 1,967 1,399 769 597 Sus- pended 360 164 622 183 676 245 232 107 Nitrogen as- Free ammo- nia. 44.4 32.5 24.7 21 31.9 43.3 38.1 37.7 Albu- minoid ammo- nia. 11.7 5.2 1.3.7 IS. 7 7 3.3 Oxygen con- simied. Total. 29 20.1 127 58.5 153 lOS 49.5 27.3 Dis- solved. 76.1 68.5 Remarks. April to Jime,1897. February, 1899, to January 15,1900. Septic tank No. 1, 1901, open tank. Tank A, January, 1898, to January, 1903. Note.—Oxygen consumed in four hours at 80° F. in the English results: in two minutes' boiling at Lav\aenoe. Solids in Lawrence figures for year 1902 only. Table XVIII shows the results obtained with an experimental tank at Brockton, Mass., in the treatment of very strong sewage pumped from the bottom of the receiving well during the last portion of the brief daily period of pumpage. The period of septic action was ■twenty-four hours. The results indicate a very high rate of purifica- tion. Boiling figures that of the suspended solids entering the tank 45 per cent remained as sludge, 6 per cent escaped in the effluent, 16 per cent went into solution, and 33 per cent disappeared as gas (Barbour, 1904). Table XVIII.—Results of treatment of sludge at Brockton, Jfoss., 1900 (Barbour, 190J^). [Parts per million.] August and September, Material. Volatile residue. Flxecjjesidue. Nitrogen as- Oxygen con- sumed. Dis- solved. In sus- pension. Dis- solved. In sus- pension. Free ammo- nia. Albuminoid ammonia. Total. Soluble. Total. Soluble. Sludge Effluent 229 285 1,810 116 271 299 555 31 34.5 44.1 42 7.5 3.9 4.6 .396 122 74.5 65.4](https://iiif.wellcomecollection.org/image/b21358205_0054.jp2/full/800%2C/0/default.jpg)