The dispensatory of the United States of America / by George B. Wood and Franklin Bache.
- Date:
- 1865
Licence: Public Domain Mark
Credit: The dispensatory of the United States of America / by George B. Wood and Franklin Bache. Source: Wellcome Collection.
Provider: This material has been provided by the National Library of Medicine (U.S.), through the Medical Heritage Library. The original may be consulted at the National Library of Medicine (U.S.)
74/1724
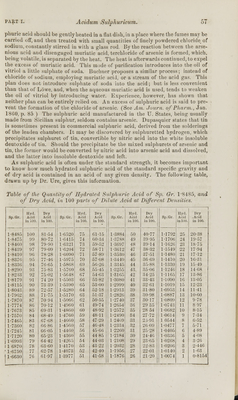
![The only way to obtain pure sulphuric acid is by distillation. Owing to the high boiling point of this acid, the operation is rather precarious, in consequence of the danger of the fracture of the retort from the sudden concussions to which the boiling acid gives rise. Dr. Ure recommends that a retort of the capacity of from two to four quarts be used in distilling a pint of acid. This is connected, by means of a wide glass tube three or four feet long, with a receiver surrounded with cold water. All the vessels must be perfectly clean, and no luting employed. The retort is then gradually heated by a small furnace of charcoal, or, what is better, by means of a sand-bath, the retort being buried in the sand up to the neck. It is useful to put into the retort a few sharp-pointed pieces of glass, or slips of platinum foil, with the view of diminishing the shocks produced by the acid vapour. The distilled product ought not to be collected until a dense gray- ish-white vapour is generated, the appearance of which is a sign that the pure concentrated acid is coming over. If this vapour should not immediately appear, it shows that the acid subjected to distillation is not of full strength; and the distilled product, until this point is attained, will be an acid water. In the dis- tillation of sulphuric acid, M. Lembertuses fragments of the mineral called quartz- ite, instead of pieces of glass or platinum foil. After a time the fragments get worn, and must be changed. The following process for purifying the acid is given in the British Pharma- copoeia. Take of Sulphuric Acid of Commerce twelve fluidounces; Sulphate of Ammonia, in powder, a quarter of an ounce [avoirdupois]. Having added the Sulphate of Ammonia to the Sulphuric Acid, introduce the mixture into a plain retort with a few slips of platinum foil, cover the upper part of the body of the retort with a sheet-iron hood, and distil over one-tenth of the acid into a flask. Remove this flask, and reject its contents; and, having applied a fresh flask, continue the distillation till only a fluidounce of liquid remains behind. Preserve the product in a stoppered bottle. Br. Composition. The hydrated ac,id of the sp. gr. 1-845 (1-846, Br., 18485, Ure) consists of one eq. of dry acid 40, and one eq. of water 9 = 49. As the water acts the part of a base, the proper name of it is sulphate of water, its formula being HO,SOr The dry acid consists of one eq. of sulphur 16, and three eqs. of oxygen 24 = 40. The ordinary commercial acid (sp. gr. 1-8433) consists, according to Phillips, of one eq. of dry acid, and one and a quarter eqs. of water. The hydrated acid of Nordhausen has a density as high as 1-89 or 1-9, and consists of two eqs. of dry acid, and one eq. of water (HO,2S03). This acid is particularly adapted to the purpose of dissolving indigo for dyeing the Saxon blue. When heated gently in a retort, connected with a dry and refrigerated receiver, dry or anhydrous sulphuric acid distils over, and the common monohydrated acid remains behind. In performing this operation, much difficulty from concussion is avoided, and the product of dry acid increased, by introducing a coil of platinum wire into the retort. The dry acid may also be obtained by the action of dry phosphoric acid on concentrated sulphuric acid, according to the method of Ch. Barreswil. The mixture must be made in a refrigerated retort, and afterwards distilled by a gen- tle heat into a refrigerated receiver. Anhydrous sulphuric acid under 64° ia in the form of small colourless crystals, resembling asbestos. It is tenacious, dif- ficult to cut, and may be moulded in the fingers like wax, without acting on them. Exposed to the air, it emits a thick opaque vapour of an acid smell. Above 64° it is a liquid, very nearly of the density 2. Medical Properties. Sulphuric acid is tonic, antiseptic, and refrigerant. In- ternally it is always administered in a dilute state. For its medical properties in this state, the reader is referred to the title, Acidum Sulphuricum Dilutum. Externally it is sometimes employed as a caustic; but, from its liquid form, it is very inconvenient for that purpose. A plan, however, has been proposed by Prof. Simpson by which it becomes very manageable. This consists in mixing it with](https://iiif.wellcomecollection.org/image/b21165282_0074.jp2/full/800%2C/0/default.jpg)