A dictionary of chemical solubilities : inorganic / by Arthur Messinger Comey.
- Comey Arthur Messinger, 1861-
- Date:
- 1896
Licence: Public Domain Mark
Credit: A dictionary of chemical solubilities : inorganic / by Arthur Messinger Comey. Source: Wellcome Collection.
Provider: This material has been provided by the Royal College of Physicians of Edinburgh. The original may be consulted at the Royal College of Physicians of Edinburgh.
37/544 (page 13)
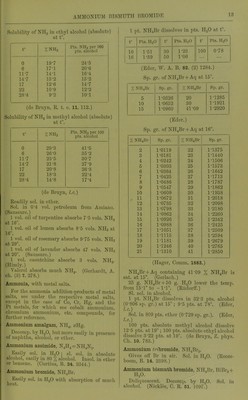
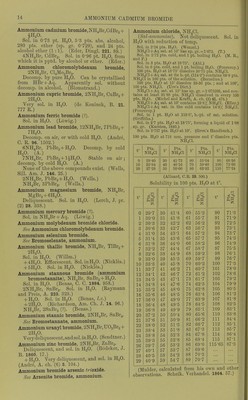
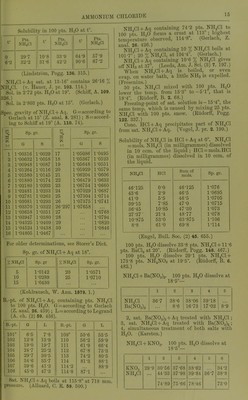
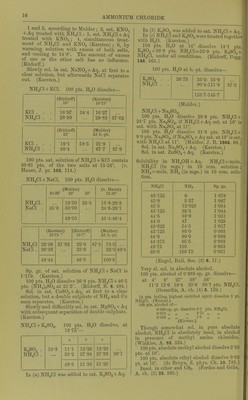
![Solubility of NH3 in ethyl alcohol (absolute) att°. ^ 0 19-7 6 17-1 117 14-1 14-7 13-2 17 12-6 •22 10-9 28-4 9-2 Pts. NHj per 100 pts. alcohol 24-5 20-6 16-4 15-2 14-7 12-2 10-1 (de Bruyn, R. t. c. 11. 112.) Solubility of NH3 in methyl alcohol (absolute) at t°. r %NH3 Pts. NH3 per 100 pts. alcohol 0 29-3 41-5 6 26-0 35-2 11-7 23-5 30-7 14-7 21-8 27-9 17 20-8 26-3 22 18-3 22-4 28-4 14-8 17-4 3 vols. NH3. (Gerhavdt, A. (de Bruyn, I.e.) Readily sol. in ether. Sol. in 0'4 vol. petroleum from Amiano. (Saussure.) 1 vol. oil of turpentine absorbs 7 5 vols. NH3 at 16°. 1 vol. oil of lemon absorbs 8*5 vols. NHs at 16°. 1 vol. oil of rosemary absorbs 9*75 vols. NH3 at 29°. 1 vol. oil of lavender absorbs 47 vols. NH3 at 20°. (Saussure.) 1 vol. caoutchine absorbs (Himly.) Valerol absorbs much NH3. ch. (3) 7. 278.) Ammonia, with metal salts. For tlie ammonia addition-products of metal salts, sec under the respective metal salts, except in the case of Co, Cr, Hg, and tlie Pt metals, for which see cobalt ammonium, cliromium ammonium, etc. compounds, for furtlicr reference. Ammonium amalgam, NH^, xHg. Deconip. by H.,0, but more easily in presence of naj)litlia, alcohol, or ether. Ammonium azoimide, N^H4 = NH4N3. Easily .sol. in H^O; si. sol. in absolute alcohol, easily in 80 % alcoliol. Insol. in ether or benzene. (Curtius, B. 24. 3344.) Ammonium bromide, NIl4Br. Easily sol. in HjO with absorption of much heat. 1 pt. NH4Br dissolves in pts. HjO at t° t° Pts. H.jO t° Pta. H...O r Pts. H-jO 10 1-51 30 1-23 100 0-78 16 1-39 50 1-06 (Eder, W. A. B. 82. (2) 1284.) Sp. gr. of NH4Br + Aq at 15°. % NH4Br Sp. gr. % NHjBr Sp. gr. 5 1-0326 20 1-1285 10 1-0652 30 1-1921 15 1-0960 41-09 1-2920 (Ed er.) Sp. gr. of NH4Br + Aq at 16°. % NH4Br Sp. gr. %NH4Br Sp. gr. 2 1-0119 22 1-1375 3 1-0181 23 1-1440 4 1-0242 24 1-1506 5 1-0303 25 1-1573 6 1-0364 26 1-1642 7 1-0425 27 1-1713 8 1-0486 28 1-1787 9 1-0547 29 1-1862 10 1-0609 30 1-1938 , 11 1-0672 31 1-2018 12 1-0735 32 1-2098 13 1-0798 33 1-2180 14 1-0862 34 1-2260 15 1-0926 35 1-2342 16 1-0988 36 1-2425 17 1-1051 37 1-2509 18 1-1115 38 1-2594 19 1-1181 39 1-2679 20 1-1246 40 1-2765 21 1-1310 41 1-2S50 (Hager, Comm. 1883.) NHjBr + Aq containing 41-09 % NH4Br is sat. at 15°. (Gerlach.) 25 g. NH4Br + 50 g. H2O lower the temp, from 15-1° to -1-1°. (Riidorif.) SI. sol. in alcohol. 1 pt. NH^Br dissolves in 32-3 pts. alcohol (0-806 sp. gr.) at 15° ; 9-5 pts. at 78°. (Eder, I.e.) Sol. in 809 pts. ether (0-729 sp. gr.). (Eder, I.e.) 100 pts. absolute methyl alcohol dissolve 12-5 pts. at 19°; 100 pts. absolute ethyl alcohol dissolve 3-22 pts. at 19°. (de Bruyn, Z. ])hys. Ch. 10.783.) y ' I y Ammonium <;-zbromide, NH^Br.,. Oives oil Br in air. Sol. in TI.,0. (Rooze- boom, B. 14. 2398.) Ammonium bismuth bromide, NH.Br, BiBr,+ ll.p. Deliquescent. Decomji. by JT.,0. Sol. in alcohol. (Nickl68, C. R. 61. 1097.)](https://iiif.wellcomecollection.org/image/b21713613_0037.jp2/full/800%2C/0/default.jpg)