A dictionary of chemical solubilities : inorganic / by Arthur Messinger Comey.
- Comey Arthur Messinger, 1861-
- Date:
- 1896
Licence: Public Domain Mark
Credit: A dictionary of chemical solubilities : inorganic / by Arthur Messinger Comey. Source: Wellcome Collection.
Provider: This material has been provided by the Royal College of Physicians of Edinburgh. The original may be consulted at the Royal College of Physicians of Edinburgh.
38/544 (page 14)
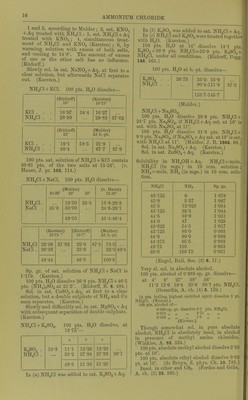
![Ammonium cadmium bromide.NH.Br,CdBin + 4H.,0. Sol. iu 0-73 pt. H2O, 5-3 pts. abs. alcohol, 280 pts. ether (sp. gr. 0729), and 24 pts. alcohol ether (1:1). (Eder, Dingl. 221. 89.) 4NH4Br, CdBrj. Sol. in 0-96 pt. HgO, from which it is pptd. by alcohol or ether. (Eder.) Ammonium chloromolybdenum bromide 2NH4Br, CljMoaBro. Decomp. by pure HjO. Can be crystallised from HBr + Aq. Apparently sol. without decomp. in alcohol. (Blomsti-and.) Ammonium cupric bromide, 2NH4Br, CuBr, + 2HoO. Very sol. in HjO. (de Koninck, B. 21. 777 R.) Ammonium ferric bromide (?). Sol. in HoO. (Lowig.) Ammonium lead bromide, 12NH4Br, 7PbBr,+ 7H2O. Decomp. on air, or with cold HjO. (Andre, C. R. 96. 1502.) 6NH4Br, PbBro + HjO. Decomp. by cold H2O. (A.) 7]SrH4Br, PbBra + HHjO. Stable on air; decomp. by cold H2O. (A.) None of the above compounds exist. (Wells, Sin. Am. J. 146. 25.) 2NH4Br, PbBra + HjO. (Wells.) NH4Br, 3PbBro. (Wells.) Ammonium magnesium bromide, NHjBr, MgBr2 + 6H.p. Deliquescent. Sol. in HjO. (Lercli, J. pr. (2) 28. 338.) Ammonium mercury bromide (?). Sol. in NH4Br + Aq. (Lii\vig.) Ammonium molybdenum bromide chloride. Sec Ammonium chloromolybdenum bromide. Ammonium selenium bromide. Sec Bromoselenate, ammonium. Ammonium thallic bromide, NH4Br, TlBr3 + 2H2O. Sol. inH20. (Willm.) + 4H2O. Efflorescent. Sol. inHgO. (Nickles.) + 5H2O. Sol. inHgO. (Nickles.) Ammonium stannous bromide (ammonium bromostannite), NHjBr, SnBra + HjO. Sol. in H.,0. (Benas, C. C. 1884. 958.) 2NH4Br, SnBro. Sol. in HjO. (Raymann and Preis, A. 223 323.) + H2O. Sol. in H2O. (Benas, Z.c.) + 2H2O. (Richardson, Am. Ch. J. 14. 96.) NHjBr, 2SnBr2 (?). (Benas.) Ammonium stannic bromide, 2NH4Br, SnBr4. Sec Bromostannate, ammonium. Ammonium uranyl bromide, 2NH4Br,U02Br2 + 2H2O. Very deliquescent, and sol. in HgO. (Sondtner.) Ammonium zinc bromide, 2NH4Br, ZnBrj. Deliquescent, and sol. iu H2O. (Bodeker, J. B. 1860. 17.) „ . + H2O. Very deliquescent, and sol. in HjO. (Andr6, A. ch. (6) 3. 104.) Ammonium bromide arsenic <? ioxide. Sec Arsenite bromide, ammonium. Ammonium chloride, NH4CI. (Sal-amvioniac). Not deliquescent. Sol. in HoO with reduction of temp. Sol. in 2-24 pts. H..0. (Wenzel.) NH4C1+Aq sat. at 10' has sp. gr. =1-072. (T.) Sol. in 2-72 pts. cold, and 1 pt. boiling HoO. (M. R., and P.) Sol. in 8 pts. H2O at 18-75°. (AM.) Sol. in 0 pts. cold, and 1 pt. boiling H2O. (Fourcroy.) 100 pts. H.jO at 18-75° dissolve 30-75 pts. NH4CI. NH4C1+Aq sat. at its b.-pt. (114-2°) contains 88-9 pts. NH4CI in 100 pts. of the solution. (Berzelius.) 100 pts. HoO at 15° dissolve 33-36 pts. : and at 100°, 100 pts. NH4CI. (Ure's Diet.) NH4C1+Aq sat. at 15° has sp. gr. =1-075209, and con- tains at least 31-88 pts. NH4CI dissolved in every 100 pts. HoO. (Michel and Kraflt, A. ch. (3) 41. 478.) NH4Cl-fAq sat. at 10° contains 23-8% NH4CI. (Eller.) NH4Cl-t-Aq sat. in the cold contains 14-3% NH4CI. (Fourcroy.) Sol. in 1 pt. HoO at 113-5°, b.-pt. of sat. solution. (Griffiths.) Sol. in 2-7 pts. H2O at 18-75°, forming a liquid of 1-08 sp. gr. (Karsten, 1840.) Sol. in 2-727 pts. HoO at 10°. (Gren's Handbuch.) 100 pts. H2O at 718 mm. pressure and t° dissolve pts. NH4CI. t° Pts. NH4CI t° Pts. NH4CI t° Pts. NH4CI t° Pts. NH4CI 0 28-40 30 41-72 60 55-04 90 68-36 10 82-84 40 46-16 70 59-48 100 72-80 20 87-28 50 50-60 SO 63-92 110 77-24 (AUuard, C.R. 59. 500.) Solubility in 100 pts. H3O at t°. t° Pts. NH4CI t° !3 t° t° I—< .0 05 -1« 0 29-7 30 41-4 60 55-2 90 71-3 1 30-0 31 41-8 61 55-7 91 71-9 2 30-3 32 42-2 62 56-2 92 72-5 3 30-6 33 42-7 63 56-7 93 73-1 4 31-0 34 43-1 64 57-2 94 73-7 5 31-4 35 43-6 65 57-7 95 74-3 6 31-8 36 44-0 66 58-2 96 74-9 7 32-2 37 44-4 67 58-7 97 75-5 8 32-6 38 44-9 68 59-2 98 76-1 9 33-0 39 45-3 69 59-7 99 76-7 10 33-3 40 45-8 70 60-2 100 77-3 11 33-7 41 46-2 71 60-7 101 78-0 12 34-1 42 46-7 72 61-2 102 78-6 13 34-5 43 47-1 73 61-7 103 79-2 14 34-8 44 47-6 74 62-3 104 79-9 15 35-2 45 48-0 75 62-8 105 80-5 16 35-6 46 48-5 76 63-4 106 81-2 17 36-0 47 49-0 77 63-9 107 81-8 18 36-4 48 49-5 78 64-5 108 82-5 19 36-8 49 49-9 79 65-1 109 83-1 20 37-2 50 50-4 80 65-6 110 83-8 21 37-6 51 50-9 81 66-2 111 84-4 22 38-0 52 51-3 82 66-7 112 85-1 23 38-4 53 51-8 83 67-3 113 85-7 24 38-8 54 52-3 84 67-8 114 86-4 25 39-3 55 52-8 85 68-4 115 871 26 39-7 56 53-2 86 69-0 115-65 87-3 27 40-1 57 53-7 87 69-6 28 40-5 58 54-2 88 70-2 29 40-9 59 54-7 89 70-7 (Mulder, calculated from his own and other observations. Scheik. Vcrhandel. 1864. 57.)](https://iiif.wellcomecollection.org/image/b21713613_0038.jp2/full/800%2C/0/default.jpg)