A dictionary of chemical solubilities : inorganic / by Arthur Messinger Comey.
- Comey Arthur Messinger, 1861-
- Date:
- 1896
Licence: Public Domain Mark
Credit: A dictionary of chemical solubilities : inorganic / by Arthur Messinger Comey. Source: Wellcome Collection.
Provider: This material has been provided by the Royal College of Physicians of Edinburgh. The original may be consulted at the Royal College of Physicians of Edinburgh.
39/544 (page 15)
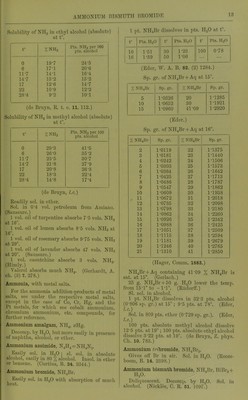
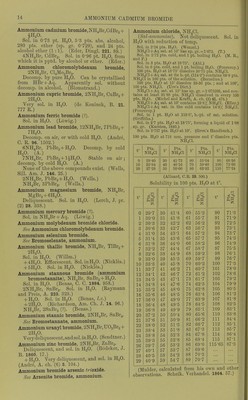
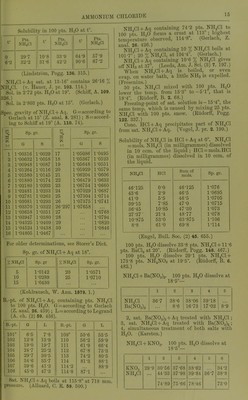
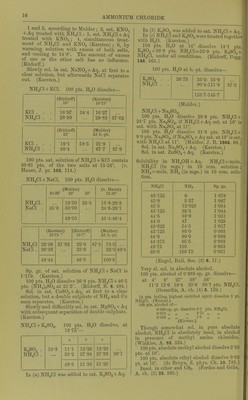
![Solubility in 100 pts. HaO at t°. f Pts. N1I4C1 t* Pts. NH4CI f Pts. NII4CI 0 6-2 29-7 32-2 10-8 31-6 33-9 42-2 64-9 90-6 57-9 67-2 (Lindstrom, Pogg. 136. 315.) NH4C1 + Aq sat. at 13-16° contains 26-16 % NH4CI. (v. Hauer, J. ]n: 103. 114.) Sol. in 2-72 pts. H.O at 19°. (SchilF, A. 109. 326.) Sol. in 2-803 pts. H2O at 15°. (Gcrlach.) Spec, gravity of NH4CI + Aq. G = according to Gerlach at 15° (Z. anal. 8. 281) ; S = accord- ing to Scliiff at 19° (A. 110. 74)^ 6 a 5^ Sp. gr. 5 w Sp. gr. G S G S 1 1-00316 1-0029 '17 1-05086 1-0495 2 1-00632 1 -0058 18 1-05367 1 -0523 3 1-00948 1-0087 ,19 1-05648 1-0551 4 1-01264 1-0116 ]20 1-05929 1-0579 5 1-01580 1-0145 |21 1-06204 1-0606 6 1-01880 1-0174 22 1-06479 1-0633 7 1-02180 1-0203 i23 1-06754 1-0660 8 1 -02481 1-0233 124 1-07029 1-0687 9 1-02781 1-0263 '25 1-07304 1-0714 10 1-03081 1-0293 26 1-07375 1-0741 11 1-03370 1-0322 26-297 1-07658 12 1-03658 1-0351 |27 1-0768 13 1 -03947 1 -0380 |28 1-0794 14 1 -04325 1-0409 29 1-0820 15 1-04524 1-0438 30 1-0846 16 1-04805 1-0467 For older determinations, see Storer's Diet. Sp. gr. of NH4C1 + Aq at 18°. %NH4C1 8p.gr % NH4CI Sp. gr. 5 1-0142 20 1-0571 10 1-0289 25 1-0710 15 1-0430 (Kohlrausch, W. Ann. 1879. 1.) B.-pt. of NH4C1 + Aq, containing pts. NH4CI to 100 pts. H2O. G = according to Gerlacli (Z. anal. 26. 439); L = according to Legrand (A. ch. (2) 69. 436). B.-pt. 0 L B.-pt. G L 101° 6-5 7-8 109° 50-6 53-5 102 12-8 13-9 no 56-2 59-9 103 19-0 19-7 111 61-9 66-4 104 24-7 25-2 112 67-8 73-3 105 29-7 30-5 113 74-2 80-5 106 34-6 35-7 114 81-3 88-1 107 39-6 41-3 114-2 88-9 108 45-0 47-3 114-8 87-1 Sat. NH4CI-I-Aq boils at 115-8° at 718 mm, pre8.sure. (Alluard, C. R. 69. 500.) NH4Cl->-Aq containing 74*2 pts. NH4CI to 100 pts IL,0 forms a crust at 113° ; highest temperature observed, 114-8°. (Gerlach, Z. anal. 26. 426.) ^^^^ ^, , , NH .01 -1- Aq containing 10 % NH4CI boils at 101 -7°; 20 % NH4CI, at 104 -4°. (Gerlacli.) NH.Cl-i-Aq containing 10-6 % NH4GI gives oir NH, at 37^ (Leeds, Am. J. Sci. (3) 7. 197.) When NHjCl-l-Aq is boiled, or even evap. on water bath, a little NH3 is expelled. (Fresenius.) 30 pts. NH4CI mixed with 100 pts. HjO lower the teni]). from 13-3° to-5-1 , that is 18-4°. (Riidorif, B. 2. 68.) Freezing-point of sat. solution is-15-4°, the same temp, which is caused by mixing 25 pts. NH4CI with 100 pts. snow. (Riidorif, Pogg. 122 337.) Cone. HCl -f Aq precipitates part of NH4CI from sat. NH4Cl-fAq. (Vogel, J. pr. 2. 199.) Solubility of NH4CI in HCl + Aq at 0°. NH4CI = mols. NH4CI (in milligrammes) dissolved in 10 ecm. of the liquid ; HCl^mols.HCl (in milligrammes) dissolved in 10 ccm. of the liquid. NH4C1 HCl Sum of inols. Sp. gr. 46-125 0-0 46-125 1-076 43-6 2-9 46-5 1-0695 41-0 5-5 46-5 1-0705 39-15 7-85 47-0 1-0715 36-45 10-85 47-30 1-073 27-37' 21-4 48-77 1-078 10-875 53-0 63-875 1-106 8-8 61-0 69-8 1-114 (Engel, Bull. Soc. (2) 45. 655.) 100 pts. H2O dissolve 33-8 pts. NH4C]-f 11-6 pts. BaCla at 20°. (Rudorff, Pogg. 148. 467.) 100 pts. H2O dissolve 29-1 pts. NH4Cl->- 173-8 pts. NH4NO3 at 19-5°. (Rudorflf, B. 6. 482.) NH4Cl-l-Ba(N03)o. 100 pts. HgO dissolve at 18-5°— 1 2 3 4 5 NH4C1 36-7 38-6 38-06 39-18 Ba(N03)2 8-6 16-73 17-02 8-9 2, sat. Ba(N03)2 -t- Aq treated with NH4CI; 3, sat. NH4CI-f-Aq treated with Ba(N03)2 ; 4, simultaneous treatment of both salts with H2O. (Karsten.) NH4CI + KNO3. 100 pts. H2O dissolve at 18-5— 1 2 8 4 6 6 KNO, NH4OI 29-9 30-56 44-33 37-68 37-98 38- 62 39- 84 36-7 34-2 38-8 74-89 75-66 78-46 73-0](https://iiif.wellcomecollection.org/image/b21713613_0039.jp2/full/800%2C/0/default.jpg)