A dictionary of chemical solubilities : inorganic / by Arthur Messinger Comey.
- Comey Arthur Messinger, 1861-
- Date:
- 1896
Licence: Public Domain Mark
Credit: A dictionary of chemical solubilities : inorganic / by Arthur Messinger Comey. Source: Wellcome Collection.
Provider: This material has been provided by the Royal College of Physicians of Edinburgh. The original may be consulted at the Royal College of Physicians of Edinburgh.
41/544 (page 17)
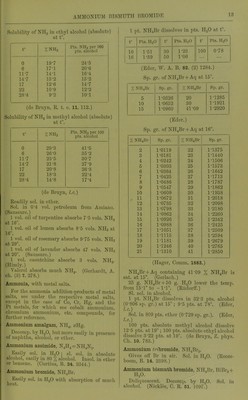
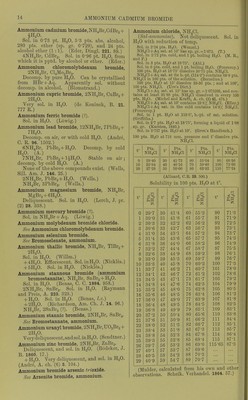
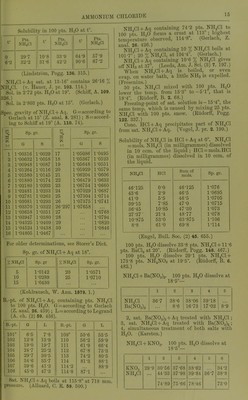
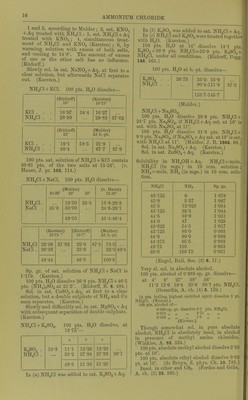
![Very si. sol. in acetone. (Krug and I'Elroy, J. anal. appl. Ch. 6. 184.) jnmonium antimonous chloride, NH^Cl, SbCis. Deliquescent. (Doluiraiu, C. R. 62. 734.) 2NH4CI, SbCl3 + 2H.30. Pernianont in dry ir; decomp. by much li^O. (Poggiale.) SNHjCl, SbCls + SHaO. As above. nunonium ajitimomc chloride, 3NH4Cl,SbCl5. Decoiup. by ILO. (DeiuTain, C. R. 62. 54.) 4NH4C1, SbClji. Decomp. by HjO. (D.) jnmonium arsenyl chloride, 2NH4CI, AsOCl + iHoO. (Wallace, Phil. Mag. (4) 16. 358.) jmnonium bismuth chloride, NH4CI, 2BiCI;,. Deliquescent. (Deheiain, C. R. 54. 724.) 2NH,C1, BiClg. Decomp. by HjO. (Arppe, •ogg. 64. 237.) + 2iHoO. (Rammelsberg.) 3NH4CI, BiCl.,. Decomp. by H2O. (Arppe.) 5NH4CI, 2BiCl3. (Rammelsberg.) Lmmonium bismuth potassium chloride, 2NH,G1, BiCls, KCl. (Deherain, C. R. 54. 724.) immonium cadmium chloride, 2NH4CI, 2CdCl2 + H20. SI. sol. in H.^0, alcohol, and wood spirit. V. Hauer, W. A. B. 13. 449.) 4XH,C1, CdCla. Sol. in HjO. (v. Hauer.) onmonium chloromolybdenum chloride, 2NH4CI, CI4M03CI0 + 2H2O. Decomp. by pure 11^0 ; can be crystallised •om HCl + Aq. (Blorasti-and.) Lmmonium chromium chloride, 2NH4CI, CrCL + H2O. 4,3 Sol. in HjO with decomp. (Neumann, A. ;44. 229.) ijnmonium cobaltous chloride, NH.Cl, CoCL + 6H2O. Deliquescent in moist air. Very easily sol. n H.,0. (Hautz, A. 66. 284.) iimnonium cobaltous chloride ammonia, NH4CI, CoCLj, NH3. (F. Rose.) Ammonium cuprous chloride, 4NH4CI, 3CU2CI2. Decomp. by HjO, not by alcohol. (Ritt- lausen, J. pr. 69. 369.) lmmonium cupric chloride, NH4CI, CuCl,+ 2II.,0. ^ Sol. in 2 pts. H2O. (Hautz, A. 66. 280.) 2NH4C!, CUCI2 + 2H2O. Easily sol. in H2O, ilso in alcohol, even when absolute. (Cai) and Henry, J. pr. 13. 184.) Ammonium cupric chloride ammonia, 2NH4CI, CuC]2, 2NH.J. Deconi]). by H2O, less easily by alcohol. Decomp. Ijy acids. (Ritthausen.) Ammonium indium chloride, 2NH4CI, InCl3 + H2O. Easily sol. in H2O. (Meyer.) Ammonium iodine chloride, NH4CI, ICI3. More sol. in H2O than KCl, ICI3. (Filhol, J. Pharm. 26. 441 ; Bcrz. J. B. 20. (2) 110.) Ammonium iridium chloride. .S't'(; Chloriridate, ammonium. Ammonium ferrous chloride, NH4CI, FeCU. Easily sol. in HqO ; insol. in alcohol. (Winkler.) Ammoniiun ferric chloride, 2NH4CI, FeCl3 + H2O. Deliquescent. Sol. in H.,0 without decomp. (Fritzsche) ; sol. in 3 pts. HoO at 1875°. (Abl.) Ammoniiun ferric potassium chloride, NH4CI, FeClg, KCl + liHaO. Min. JCremcrsitc. Deliquescent, Ammonium lead chloride, NH4CI, 2PbCl2 + 3H2O. Sol. in HoO without decomp, (?). (Andr6, C. R. 96. 1502.) 6NH4CI, PbCl2+H„0. 9NH4CI, PbCl2 + liH20. 9NH4CI, 2PbClo + 2iH20. IONH4CI, PbCl2 + H20. llNH4Cr, 2PbCl2 + 3iH20. I8NH4CI, PbCl2 + 4H20. All these salts are decomp, by HoO. (Andr(5, A. ch. (6) 3. 104.) Of the salts prepared by Andrd, only one exists, NH4CI, 2PbCl2. (Wells, Sill. Am. J. 146. 25.) NH4CI, PbClj + JHaO. (Wells, I.e.) Ammonium lead tetrachloride. Sec Chloroplumbate, ammonium. Ammonium magnesium chloride, NH4MgCl3 + 6H20 = NH4C1, MgCla + eHgO. Deliquescent. Very sol. in HoO. Sol. in 6 pts. cold HoO. (Fourcroy.) 4NH4CI,'5MgCl„ + 33H„0. Sol. in HoO. (Berthelot and And're, A. ch. (6) 11. 294.) Ammonium manganous chloride, NH4CI, MnCl2 + iH20. Sol. in 1^ pts. HjO at ordinary temp. (Hautz, A. 66. 280); does not exist. (Saunders, Am. Ch. J. 14. 134.) 2NH4CI, MnCla + HaO. Sol. in HjO (Ram- melsberg) ; does not exist. (Saunders.) + 2H2O. Easily sol. in HjO, but with decomp. into NH4CI and MnClj. (Saunders.) Ammonium mercuric chloride, 2NH4CI, HgCla + H2O (sal alembroth). Sol. in 0-66 pt. HjO at 10°, and in nearly every i)roportion of hot HoO. NH4CI, HgClo. Easilyaol. in H^O. + Easily sol. in H2O. (Kane.) 2NH4CI, 3HgCl2 + 4H20. Easily sol. in H2O. (Holmes, C, N. 6. 351.) 2NH4CI, 9HgCl2. Sol. inH20. (Holmes.)](https://iiif.wellcomecollection.org/image/b21713613_0041.jp2/full/800%2C/0/default.jpg)