A dictionary of chemical solubilities : inorganic / by Arthur Messinger Comey.
- Comey Arthur Messinger, 1861-
- Date:
- 1896
Licence: Public Domain Mark
Credit: A dictionary of chemical solubilities : inorganic / by Arthur Messinger Comey. Source: Wellcome Collection.
Provider: This material has been provided by the Royal College of Physicians of Edinburgh. The original may be consulted at the Royal College of Physicians of Edinburgh.
450/544 (page 426)
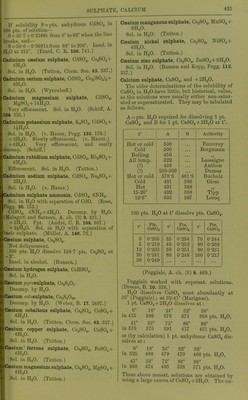
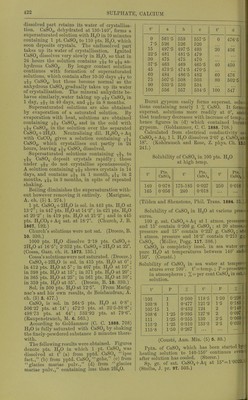
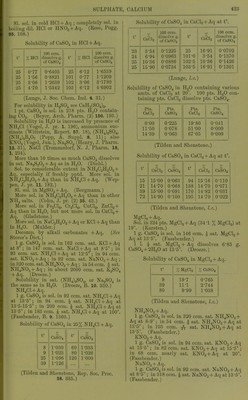
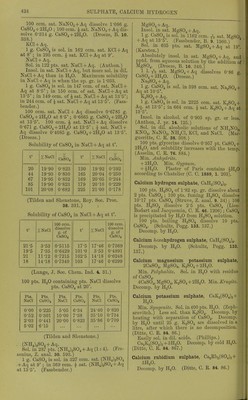
![by further addition of ILfl, which redissolves on evaporation. SCroOg, I2SO3 (?). (Siewert.) Chromic sulphate, 01-2(804)3. Anhydrous. Insol. in H2O, HNO3, HCl, H2SO4, aquaregia, andNH^OH + Aq. Decomp. by boiling caustic alkalies, and slowly by alkali carbonates+ Aq. (Sclirbtter.) Accord- ing to Traube (A. 71. 92) and Siewert (A. 126. 94), Schrbtter's salt is an acid sulphate, Cr4(S04)5(OS,020H)2 = 2Cr2(S04)3, H2SO4. Ac- cording to Etard (Bull. Soc. (2) 31. 200) both salts exist, and formula of above salt is Cr2(S04)6Cr2. Formula is 2[(Cr203)2, (SOa)^], 17H0SO4 (?). (Cross and Higgins, Chem. Soc. 41. 113.) -t-CHjO (?). Green modification. Readily sol. in H2O or alcohol. Sol. in cone. H2SO4. H2O solution is converted into the violet modi- fication by standing 3-4 weeks. (Schrotter.) + IIH2O (?). Extremely deliquescent; be- comes liquid in moist air in 2 minutes. Not pptd. by BaCLj + Aq. (Recoura, C. R. 113. 857.) -flSHjO. Violet modification. Sol. in 0833 pt. H2O at 20°. When the H2O solution is heated to 65-70° it begins to be converted into the green modification. This conversion is also brouglit about by cold HNO3, H2SO4, PCI3. (Etard, C. R. 84. 1090.) Sp. gr. of aqueous solution of violet modi- fication of Cr2(S04)3 containing: 5 10 20 % 01-2(804)3-flSHaO, 1-0275 1-0560 1-1150 30 40 50 % 0r2(8O4)3 + 18H2O. 1-1785 1-2480 1-3250 Sp. gr. of aqueous solution of green modifica- tion of Or2(804)3 containing: 10 20 30 % 0^2(804)3-H8H2O, 1-0510 1-1070 1-1680 40 50 60 % Or2(S04)3-H8H20, 1-2340 1-3055 1-3825 70 80 % Or2(S04)3-fl8H20. 1-4650 1-5535 (Gerlach, Z. anal. 28. 494.) See also Chromosulphuric acid. Chromic sulphate, acid, 2Cr2(S04)3, 112804= p (0S020H)2 (804)5. Correct composition of Cr2(S04)3 (Traube), which see. See also Chromosulphuric acid. Chromic cupric sulphate, Cr2(S04)3, 2OUSO4, H28O4. Insol. in HgO, but gradually decomp. there- by. (Etard, 0. R. 87. 602.) Chromic ferrous sulphate, Cr2(804)3, 2Fe804, H2S04-i-2H-jO. As above. (Etard, I.e.) Chromic ferric sulphate, 010(804)3, Fe2(804)3. Insol. in H2O. (Etard, C. R. 86. 1399.) Cr2(S04)3, Fe2(S04)3, H2SO4. Insol. in H^O. (Etard.) ^ Chromic hydroxylamine sulphate, Cr,(SOj). (NH.20H)2S04 + 24H20. Sol. inH20. (Meyeringh.) Chromic lithium sulphate, Cr2(S04)3, 3Li2S04. Resembles the corresponding K salt. ( Wer- nicke.) Chromic manganous sulphate, Cr„(SO,), 3MnS04. (Etard, 0. R. 86. 1402.) Chromic manganic sulphate, Cro(SO,)». Mn2(804)3. ^ Insol. in HoO. (Etard, 0. R. 86. 1399.) Cr2(S04)3, Mn2(S04)3, 2H2SO4. 81., deliques- cent. Sol. in HoO with decomp. (Etard.) Chromic nickel sulphate, 0ro(804)o, NiSO,, 2H„S04-f3HoO. Insol. in HoO, but gradually decomp. there- by. (Etard, C. R. 87. 602.) Chromous potassium sulphate, CrS04, K2S04-H 6 HoO. Sol. in HoO ; less sol. in alcohol. (Peligot, A. ch. (3) 12. 546.) Chromic potassium sulphate, I£20r2(804)4. Anhydrous, a. Sol. in H2O when not heated over 350°. /3. Insol. in cold HjO and cold acids. Wiien ignited is insol. in hot HoO and acids, except slightly in boiling cone. H0SO4. (Fischer.) -f2H20 (?). Insol. in cold HjO or dil. acids. Sol. by long boiling witli HoO, and niori quickly when HOI is added. (Hertwig.) -t-4H20. Is potassium chromosul^ihate, which see. -f 24 HoO. Chrome-alum. Violet modification. Effloresce'nt at 29°. Sol. in 6-7 pts. cold HjO. When the HoO solution is heated to 60-70° it is partially decomp. into a green modification, which is more sol. in HjO. The green modi- fication on standing in H2O solution is very slowly converted back into violet modification. The green modification may also be formed by heating dry salt to 100°, at which temp, it melts in its crystal HgO. When all crystal H2O has been expelled at 300-350°, it still dis solves in hot H2O, but when heated above 350 it becomes insol. in HjO. (Lbwel, A. ch. (3) 44. 313.) Insol. in alcohol. Melts in crystal H2O at 89°. (Tilden, Chem. Soc. 45. 409.) Sp. gr. of chrome-alum solutions at 15° con- taining : 5 10 15 20 25 %salt, 1-0174 1-0342 1-0524 1-0746 1-1004 30 35 40 45 50 % salt, 1-1274 1-1572 1-1896 1-2352 1-2894 55 60 65 70 % salt 1-3704 1-4566 1-5462 1-6362 (Fi-anz, J. pr. (2) 5. 298.) It,](https://iiif.wellcomecollection.org/image/b21713613_0450.jp2/full/800%2C/0/default.jpg)