A dictionary of chemical solubilities : inorganic / by Arthur Messinger Comey.
- Comey Arthur Messinger, 1861-
- Date:
- 1896
Licence: Public Domain Mark
Credit: A dictionary of chemical solubilities : inorganic / by Arthur Messinger Comey. Source: Wellcome Collection.
Provider: This material has been provided by the Royal College of Physicians of Edinburgh. The original may be consulted at the Royal College of Physicians of Edinburgh.
451/544 (page 427)
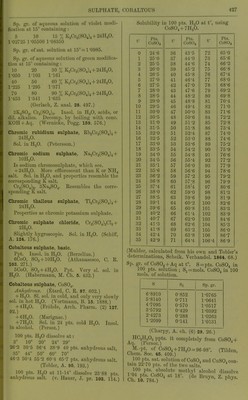
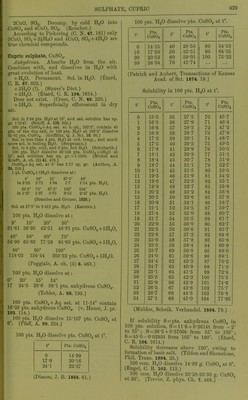
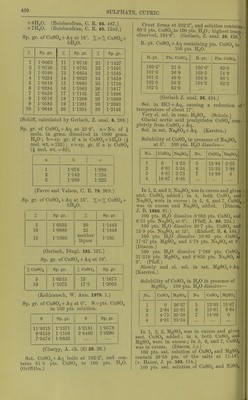
![Sp. gr. of aqueous solution of violet niodi- ficatiou at 16° containing : 6 10 If) % K„Cr2(S04)4 + 24HaO. 1 •02725 1-05500 1-08350 Sp. gr. of sat. solution at 15°=1-0985. Sp. gr. of aqueous solution of green modifica- tion at 15 containing : 10 20 30 % KoCr2(S04)4 + 24H20, 1-050 1-103 1-161 40 50 60 % K2Cr„(S04)4 + 24H20, 1-2-25 1-295 1-371 70 80 90 % K„Cro(S04)4 + 24H20. 1-453 1-541 1-635 (Gerlach, Z. anal. 28. 497.) SK^Oj, CrjCSOj);,. Insol. in HgO, acids, or dil. alkalies. Decomp. by boiling with cone. KOH + Aq. CWernicke, Pogg. 169. 576.) Chromic rubidium sulphate, RbjCrjCSOj)^+ •24H.p. Sol. in HoO. (Petersson.) Chromic sodium sulphate, Na2Cr2(S04)4+ IOH2O. Is sodium chromosulphate, which see. + 24H0O. More efflorescent than K or NH4 salt. Sol. in H,0, and properties resemble the corresponding K salt. Cr2(S04)3, 3Na^S04. Resembles the corre- sponding K salt. Chromic thallous sulphate, Tl2Cr„(S04)4-h 24H2O. Properties as chromic potassium sulphate. Chromic sulphate chloride, Cro(S04)2Cl„-l- 2H2O. Slightly hygroscopic. Sol. in H2O. (Schiff, A. 124. 176.) Cobaltous sulphate, basic. P])t. Insol. in HoO. (Berzelius.) 6C0O, SO3-HOH2O. (Athanasesco, C. R. 103. 271.) 5CoO, S03-f4H20. Ppt. Very si. sol. in H2O. (Habermann, M. Ch. 6. 432.) Cobaltous sulphate, C0SO4. Anhydrous. (Ktard, C. R. 87. 602.) -t-HjO. SI. sol. in cold, and only very slowly sol. in hot H2O. (Vortmann, B. 16. 1888.) -f4H20. (Frohde, Arch. Pharm. (2) 127. 92.) -t-6H20. (Marignac.) -f-7H20. Sol. in 24 pts. cold H2O. Insol. in alcohol. (Persoz.) 100 pts. H2O dissolve at: 2, 10° 20° 24° 29° 26-2 30-5 36-4 38-9 40 pts. anhydrous .salt, 35° 44° 50° 60° 70° 46-3 50-4 55-2 60-4 65-7 pts. anhydrous salt. (Tobler, A. 96. 193.) 100 pts. H2O at 11-14° dissolve 23-88 pts. anhydrous salt. (v. Hauer, J. pr. 103. 114.) Solubility in 100 pts. H2O at t°, using CoS04-<-7H20. t° Ptfi. t° n J 1 LH. f Pf a C08O4 C0SO4 C0S04 0 24-6 36 43-5 72 65-0 1 25-0 37 44-0 73 65-6 2 25-5 38 44-6 74 66-2 3 26-0 39 45-2 75 66-8 4 26-5 40 45-8 76 67-4 5 27-0 41 46-4 77 68-0 6 27-5 42 47-0 78 68-6 7 28-0 43 47-6 79 69-2 8 28-5 44 48-2 80 69-8 9 29-0 45 48-8 81 70-4 10 29-5 46 49-4 82 71-0 11 30-0 47 50-0 83 71-6 12 30-5 48 50-6 84 72-2 13 31-0 49 51-2 85 72-8 14 31-5 50 51-8 86 73-4 15 32-0 51 52-4 87 74-0 16 32-5 52 53-0 88 74-6 17 33-0 53 53-6 89 75-2 18 33-5 54 54-2 90 75-9 19 34-0 55 54-8 91 76-6 20 34-5 56 55-4 92 77-2 21 35-1 57 56-0 93 77-9 22 35-6 58 56-6 94 78-6 23 36-2 59 57-2 95 79-2 24 36-8 60 57-8 96 79-9 25 37-4 61 58-4 97 80-6 26 38-0 62 59-0 98 81-3 27 38-5 63 59-6 99 81-9 28 39-1 64 60-2 100 82-6 29 39-6 65 60-8 101 83-3 30 40-2 66 fil -4 00 y 31 40-7 67 62-0 103 84-6 32 41-3 68 62-6 104 85-3 33 41-8 69 63-2 105 86-0 34 42-4 70 63-8 106 86-7 35 42-9 71 64-4 106-4 86-9 (Mulder, calculated from his own and Tobler's determinations, Scheik. Verliandel. 1864. 68.) Sp. gr. of CoS04-^Aq at t°. S = pts. C0SO4 in 100 pts. solution ; Si=mols. C0SO4 in 100 mols. of solution. s Si Sp. gr. 6-8910 0-852 1-0765 5-8140 0-711 1-0641 4-7095 0-570 1-0517 3-5792 • 0-429 1-0392 2-4273 0-288 1-0263 1-2099 0-141 1-0131 HC2H3O2 ppts. it completely from CoSOj -f An. (Persoz.) M.-pt of CoS04-f7H20 = 96-98°. (Tilden, Chem. Soc. 46. 409.) 100 i)ts. sat. solution of C0SO4 and CuSO. con- tain 22-70 pts. of tiic two salts. T fl^CO''! Ciissolvo CI?; 10 7840](https://iiif.wellcomecollection.org/image/b21713613_0451.jp2/full/800%2C/0/default.jpg)