A dictionary of chemical solubilities : inorganic / by Arthur Messinger Comey.
- Comey Arthur Messinger, 1861-
- Date:
- 1896
Licence: Public Domain Mark
Credit: A dictionary of chemical solubilities : inorganic / by Arthur Messinger Comey. Source: Wellcome Collection.
Provider: This material has been provided by the Royal College of Physicians of Edinburgh. The original may be consulted at the Royal College of Physicians of Edinburgh.
461/544 (page 437)
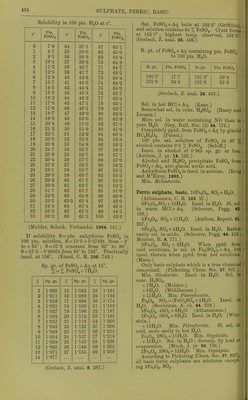
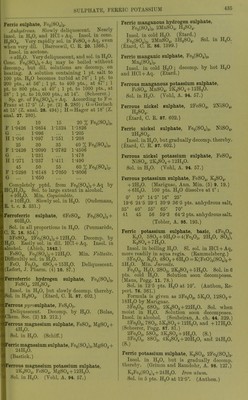
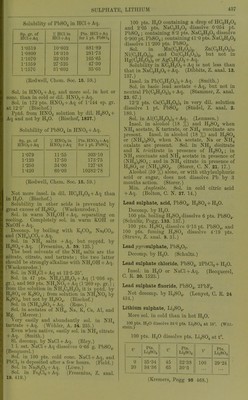
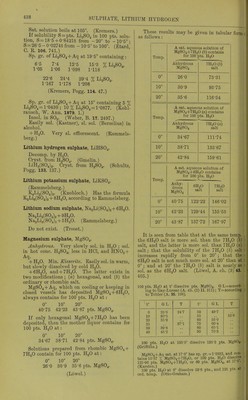
![Solubility of PbSOj in HCl + Aq. Sp. gr. of % HCl in lifjlj. An Pts. HCl+Aq fnr 1 lit PbSO 1 1 0ol9 001 oy 1 -osoo 16-310 281-73 1-1070 22-010 105-65 1-1359 27-525 47-30 1-1570 31-602 35-03 (Rodwell, Chem. Soc. 16. 59.) Sol. ill HNOg + Aq, and more sol. in hot or cone, than in cold or dil. HNO3 + Aq. Sol. in 172 pts. HNOj + Aq of 1-144 sp. gr. at 12-5' (Bischof.) Pptd. from HNO3 solution by dil. H.2SO4 + Aq and not by H2O. (Bischof, 1827.) SolubiUty of PbS04 in H]Sr03 +Aq. Sp. gr. of HNOs+Aq X HNOs in HNOs+Aq Pts.HNOs+Aq for 1 pt. PbSOj 1-079 11-55 303-10 1-123 17-50 173-75 1-250 34-00 127-48 1-420 60-00 10282-78 (Rodwell, Chem. Soc. 16. 59.) Not more insol. in dil. HC2H302+Aq than inHaO. (Bischof.) Solubility in otlier acids is prevented by great excess of H2SO4. (Wackenroder.) Sol. in warm NH40H+Aq, separating on cooling. Completely sol. in warm KOH or NaOH + Aq. Decomp. by boiling with K2CO.,, NaoCO-,, and (NH4)2C03 + Aq. Sol. in NH4 salts +Aq, but repptd. by H2S04 + Aq. (Fresenius, A. 69. 125.) Tlie best solvents of the NH4 salts are the nitrate, citrate, and tartrate ; the two latter should be strongly alkaline with NH40H + Aq. (Wackenroder.) Sol. in NH4C1 + Aq at 12-5-25°. Sol. in 47 pts. NH4C2H302 + Aq (1-036 sp. gr.), and 969 pts. NH4NO3 +Aq (1 -269 sp. gr.) ; from the solution in NH^C^HaOa it is pptd. by H2SO4 or K2SO4; from solu'tion in NH4NO3 by K2SO4, but not by H2SO4. (Bischof.) Sol. in (NH4)2S04 + Aq. (Rope.) Sol. in acetates of NH4, Na, K, Ca, Al, and Mg. (Mercer.) Very easily and abundantly sol. in NH4 tartrate +Aq. (Wohler, A. 34. 235.) Even when native, easily sol. in NH4 citrate + Aq. (Smith.) SI. decomp. byNaCl + Aq. (Bley.) 1 1. sat. NaCl + Aq dissolves 0-66 g. PbS04. (Becquerel.) Sol. in 100 pts. cold couc. NaCl + Aq, and PbCl.^ is deposited after a few hours. (Field.) Sol. in Na2S20.j + Aq. (Lowe.) Sol. in Fe^Clg + Aq. (Fresenius, Z. anal. 19. 419.) 100 i)ts. H„0 containing a drop of HC2H3O2 and 2-05 pts. 'NaC^HPi dissolve 0-054 pt. PbS04; containing 8-2 pts. NaC2H302 dissolve 0-900 pt. PbS04; containing 41 -0 pts. NaCaHaOa dissolve 11 -200 jits. PbS04. Sol. in Mn(C2H,,02)2, Zn(C2H302),, Ni(CoH302)2, and Cu(C2H302)2, but not in Hg(C2H302)2 or AgCsH-A + Aq. Solubility in KC2H302 + Aq is not less than that in NaCgHsOa + Aq. (Dibbits, Z. anal. 13. 137.) Insol. in Pb(C.,H302)2 + Aq. (Smith.) Sol. in basic lead acetate +Aq, but not in neutral Pb(C2H302)2 + Aq. (Stammer, Z. anal. 23. 67.) 12-2 pts. Ca(C2H302)2 in very dil. solution dissolve 1 pt. PbS04. (Stiidel, Z. anal. 2. 180.) Sol. in A1(C2H302)3 + Aq. (Lennsen.) Insol. in alcohol (18 %) and H2SO4 when NH4 acetate, K tartrate, or NH4 succinate are present. Insol. in alcohol (18 %) and H2SO4 or (NH4)2S04 when Na acetate, Na or NH4 oxalate are present. Sol. in NH4 cJtcitrate and K iricitta,te in presence of H2SO4; in NH4 succinate and NH4 acetate in presence of (NH4)2S04; and in NH4 citrate in presence of H2SO4 or (NH4)2S04. (Storer, C. N. 21. 17.) Alcohol (59 %) alone, or with ethylsulphuric acid or sugar, does not dissolve Pb by 3 months action. (Storer.) Min. Ancjlcsitc. Sol. in cold citric acid + Aq. (Bolton, C. N. 37. 14.) Lead sulphate, acid, PbS04, H2SO4 + H2O. Decomp. by HgO. 100 pts. boiling H2SO4 dissolve 6 pts. PbS04. (Schultz, Pogg. 133. 137.) 100 pts. H2SO4 dissolve 0-13 pt. PbS04, and 100 pts. fuming H2SO4 dissolve 4-19 pts. (Struve, Z. anal. 9. 31.) Lead j/jj/rosulphate, PbS207. Decomji. by HjO. (Schultz.) Lead sulphate chloride, PbS04, 2PbCl2 + H20. Insol. in H2O or NaCl + Aq. (Becquerel, C. R. 20. 1523.) Lead sulphate fluoride, PbS04, 2PbF2. Not decomp. by H2SO4. (Lonyet, C. R. 24 434.) Lithium sulphate, Li,2S04. More sol. in cold than in hot H2O. 100 pts. H3O dissolve 84-C pts. Li2804 at 18°. (Witi- steiu.) 100 pts. H2O dissolve pts. Li2S04 at t°. f Pts. Li2S04 t' Pts. Li2S04 f Pts. 0 20 35-34 34-36 45 65 32-38 30-3 100 29-24 (Kremers, Pogg 96 468.)](https://iiif.wellcomecollection.org/image/b21713613_0461.jp2/full/800%2C/0/default.jpg)