A dictionary of chemical solubilities : inorganic / by Arthur Messinger Comey.
- Comey Arthur Messinger, 1861-
- Date:
- 1896
Licence: Public Domain Mark
Credit: A dictionary of chemical solubilities : inorganic / by Arthur Messinger Comey. Source: Wellcome Collection.
Provider: This material has been provided by the Royal College of Physicians of Edinburgh. The original may be consulted at the Royal College of Physicians of Edinburgh.
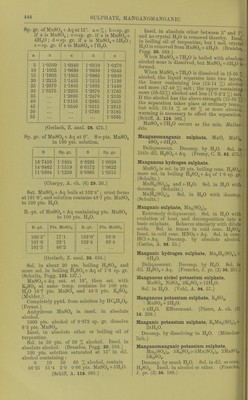
465/544 (page 441)
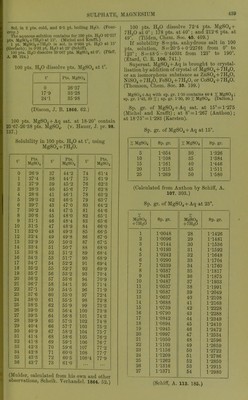
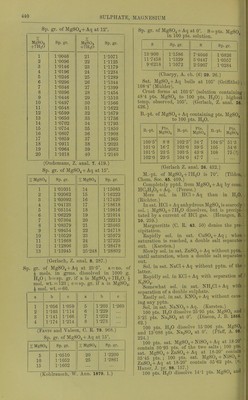
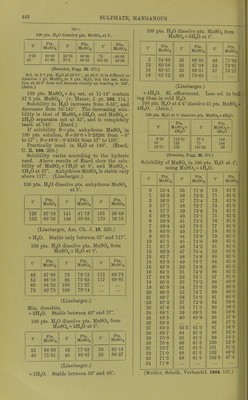
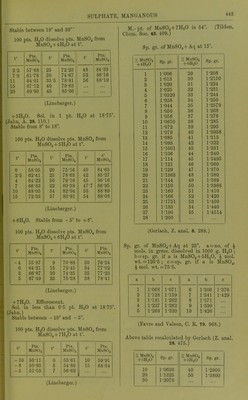
![9-8 pta. K.JSO4, if sat. MgSOj + Aq is sat. with K^Oj ; 32-4 pts. MgSO^ ami 8-'2 pts. K2SO4, if sat. K„S04 + Aq is sat. with MgSOj, all at 15. (Mulder, J. B. 1866.) 100 pts. dil. alcohol coutoining : 10 20 40 % alcohol contain at 15°, 39-3 21-3 1-62 % MgS04 + 7H20. (Schiff, A. 118. 365.) At higher temp, the solubility increases proportional to the temp. (Gerardin, A. ch. (4) B. 145.) 100 pts. absolute methyl alcohol dissolve 1-lS pts. MgSOj at 18°. (de Bruyn, Z. phys. Ch. 10. 783.) 100 pts. absolute methyl alcohol dissolve 41 pts. MgS04 + 7H„0 at 17°; 100 pts. absolute methyl alcohol dissolve 29 pts. MgS04 + 7H2O at 3-4° ; 100 pts. 93 % methyl alcohol dissolve 9-7 pts. MgSO + 7H20 at 17°; 100 pts. 50 % methyl alcohol dissolve 4'1 pts. MgS04 + 7H20 at 3-4°. (de Bruyn, R. t. c. 11. 112.) 100 pts. absolute ethyl alcohol dissolve 1*3 pts. MgS04 + 7H,,0 at 3°. (de Bruyn.) Magnesium hydrogen sulphate, MgH2(S04)2. Decomp. by II2O. Sol. in H„S04. MgHg(S04)4. Boiling H2SO4 dissolves about 2 % MgS04, from which this compound crystal- lises. (Schultz, Pogg. 133. 137.) Magnesium jji/rosulphate, MgS207. Decomp. by H2O. Magnesiiun manganous sulphate, MgSOj, 2MuS04+15H„0. Min. Fauserite. Magnesium manganous zinc sulphate, MgSOj, MUSO4, ZnS04 + 2lH20. Sol. in HoO. (Vohl, A. 99. 124.) Magnesium nickel sulphate, MgS04, 3MS0. -f 28H2O. Sol. in H2O. (Schiff.) Magnesium nickel potassium sulphate, MgSOj, ]SiS04, 2X2804-}-12H.p. Sol. in H2O. (Vohl, A. 94. 57.) Magnesium potassium sulphate, MgKo(SO.), -f- 6H2O. 100 pts. H2O dissolve 227 pts. anhydrous salt at 16-5'. (Mulder.) 100 pts. HjO dissolve at: 0° 10° 20° 30° 35° 14-1 19-6 25-0 30-4 33-2 pts. anhydrous salt, 45° 55° 60° 65° 76° 40-5 47-0 50-2 53-0 59-8 pts. anhydrous salt. (Tobler, A. 96. 193.) Sp. gr. of aqueous solution at 15° contain- ing: 2 4 6 8 % hydrous salt, 1-0129 1-0261 1-0394 1-053 10 12 14 1-0668 1-0808 1-095 18 20 22 % hydrous salt. 1-124 1-1388 1-1539 (Schiff, A. 113. 183, calculated by Gerlach, Z. anal. 8. 287.) 16 % hydrous salt, 1-1094 Min. Picromerite. + (van der Heide, B. 26. 414.) Magnesium potassium zinc sulphate, MgS04, 2K2SO4, ZnS04-t-121120. Sol. in H2O. (Vohl, A. 94. 57.) Magnesium potassium sulphate chloride, MgS04, 1^,804, MgCla-feHaO. Min. Kainite. Magnesium rubidium sulphate, MgS04, Rb2S04-F6H20. Sol. in H2O. (Tuttou, Chem. Soc, 63. 337.) Magnesium sodiujn sulphate, MgS04, NaoS04 -f 4H2O. Min. Blodite, Simonyite. Blodite is efflorescent; Simonyite deli- quescent. -f5H20. Min. Lowite. -I-6H2O. Decomp. on air. Sol. in 3 pts. cold H2O. Magnesium thallous sulphate, MgS04, TI2SO4 -t- 6H2O. Sol. in HoO, but decomp. by repeated re- crystallisations. (Werther.) Magnesium zinc sulphate, MgS04, ZnS04-l- Sol. in H2O. (Pierre, A. ch. (3) 16. 244.) + IOH2O. (Pierre.) 3ZnS04, 5MgS04-t-56H20. (Schiff.) Magnesium sulphate potassium chloride, MgS04, KCl-h3H20 or MgSOj, IV0SO4, MgCla + eHjO. Min. Kainite. 100 pts. H2O dissolve 79-56 pts. at 18°. (Krause, Arch. Pharm. (3) 6. 326.) Not sol. in a mixture of abs. alcohol and ether, which dissolves out MgCl2. (Lehmann, J. B. 1867. 416.) Alcohol dissolves out MgOlj, also little HgO. Much HoO dissolves completely. (Zincken, Miner. Jahrb. 1866, 310.) Magnesium sulphate potassium chromate, 2MgS04, K2Cr04-f9H20. Sol. in HgO. (Etard, C. K 86. 443.) Manganous sulphate, basic, 3MuO, 2S0,-t- 3H2O. lusol. in H2O, but slowly decomp. thereby. (Gorgeu, 0. R. 94. 1425.) Manganous sulphate, MUSO4. Anhydrous. Absorbs HoO from the air to form MiiS04-t-4n20. 1 pt. M11SO4 is sol. in pts. HaO at t°. t° Pts. HoO t° Pts. HgO f Pts. H2O 6-25 10 1-77 1M)81 18-75 37-5 1-607 1-457 76 101 25 1- 4!)4 2- 031](https://iiif.wellcomecollection.org/image/b21713613_0465.jp2/full/800%2C/0/default.jpg)