A dictionary of chemical solubilities : inorganic / by Arthur Messinger Comey.
- Comey Arthur Messinger, 1861-
- Date:
- 1896
Licence: Public Domain Mark
Credit: A dictionary of chemical solubilities : inorganic / by Arthur Messinger Comey. Source: Wellcome Collection.
Provider: This material has been provided by the Royal College of Physicians of Edinburgh. The original may be consulted at the Royal College of Physicians of Edinburgh.
472/544 (page 448)
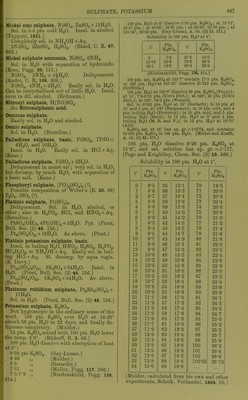
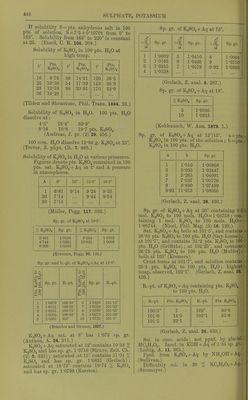
![If solubility S = pts. anhydrous salt in 100 pts. of solution, S = 7-5+0-1070t from 0° to 163°. Solubility from 163° to 220° is constant at 25. (Etard, C. R. 106. 208.) Solubility of K2SO4 in 100 pts. HgO at high temp. t Pts. K2SO4 t' Pts. K2SO4 t° Pts. K2SO4 16 9-76 39 14-21 120 26-5 20 10-30 54 17-39 143 28-8 28 12-59 98 23-91 170 32-9 36 13-28 (Tilden and Shenstone, Phil. Trans. 1884. 23.) Solubility of K2SO4 in HgO. 100 pts. HgO dissolve at: 4-3° 18-4° 69-9° 8-16 10-8 19-7 pts. K0SO4. (Andreae, J. pr. (2) 29. 456.) 100 ccm. H2O dissolve 12-04 g. K2S04at 25°. (Ti-evor, Z. phys. Ch. 7. 468.) Solubility of K2SO4 in HgO at various pressures. Figures denote pts. K2SO4 contained in 100 pts. sat. K2S04H-Aq at t° and A pressure in atmospheres. A 0° 15° 15-5° 16-2° 1 6-81 9-14 9-24 9-35 20 7-14 9-44 9-54 30 7-14 (Holier, Fogg. 117. 386.) Sp. gr. of K2SO4 at 19-5°. % K2S04 Sp. gr. % K2SO4 Sp. gr. 2-401 4-744 6-968 1-0198 1-0385 1-0568 9-264 10-945 1-0763 1-0909 (Kremers, Pogg. 95. 120.) Sp. gr. and b.-pt. of K2S04-|-Aq at 12-5°. So -r « p. *J 0 Sp. gr. B.-pt. Pts. K0SO4 to 100 pts. H2O Sp. gr. B.-pt. 1 1-0079 100-38° 6 1-0456 101-12° 2 1-0151 100-63° 7 1-0524 101-25° 3 1-0231 100-75° 8 1-0599 101-25° 4 1-0305 100-88° 9 1-0076 101-38° 5 1-0391 101° 10 1-0735 101-5° (Braiides and Gruner, 1827.) KoS04 + Aq sat. at 8° has 1-072 sp. gr. (Anthon, A. 24. 211.) K2S04-f Aq saturated at 12° contains 10-38 % K2SO4 and has sp. gr. 1-0716 (Sti'uve, Zeit. Ch. (2) 5. 323) ; saturated at 15° contains 11-01 % K2SO4 and has sp. gr. 1-0831 (Gerlach) ; saturated at 18-75° contains 10-74 % K2SO4 and has sp. gr. 1-0798 (Karsten). Sp. gr. of K2S04-f Aq at 15°. 0 t« Sp. gr. w Sp. gr. 0 « Sp. gr. 1 1-0082 5 1-0410 8 1 -0664 2 1-0163 6 1-0495 9 1-0750 3 1-0245 7 1-0579 9-92 1-0830 4 1-0328 (Gerlach, Z. anal. 8. 287.) Sp. gr. of K2S04-fAq at 18°. 7o K2SO4 Sp.gr. 5 10 1-0395 1-0815 (Kohlrausch, W. Ann. 1879. 1.) Sp. gr. of K;2S04-t-Aq at 15°/]5°. a = pts. K2SO4 in 100 pts. of the solution ; b = pts. K2SO4 in 100 pts. H2O. a b Sp.gr. 1 1-010 1-00808 3 3-093 1-02447 5 5-263 1-04091 7 7-527 1-05776 9 9-890 1-07499 9-92 11-013 1-08305 (Gerlach, Z. anal. 28. 493.) Sp. gr. of K2S04 + Aq at 20° containing O . mol. K2SO4 to 100 mols. H2O = l-03758; con- taining 1 mol. K2SO4 to 100 mols. H20 = 1-06744. (Nicol, Phil. Mag. (5) 16. 122.) Sat. ICoSOj-fAq boils at 101-5°, and contains 26-33 pts. K2SO4 to 100 pts. H2O (Gay-Lussac): at 101-7°, and contains 21-2 pts. K2SO4 to 100 pts. HoO (Griffiths) ; at 102-25°, and contains 26-75 pts. K2SO4 to 100 pts. H2O (Mulder): boils at 103° (Kremers). Crust forms at 101-7°, and solution contains 25-3 pts. K2SO4 to 100 pts. H2O; highest temp, observed, 102-1°. (Gerlach, Z. anal. 26. 426.) B.-pt. of K2S04-l-Aq containing pts. K2SO4 to 100 pts. H2O. ■ B.-pt. Pts. K2SO4 B.-pt. Pts. KoSO , 100-5° 7 102° 30-0 101-0 14-5 102-1 31-6 101-5 22-1 (Gerlach, Z. anal. 26. 430.) Sol. in cone, acids ; not pptd. by glacial HC2H3O2. Insol. in KOH-l-Aq of 1-35 sp. gi. (Liebig, A. 11. 262.) Pptd. from KoS04-hAq by NH40H-t-Aq. (Sullivan.) Difficultly sol. in 20 % KCaHsOg + Aq. (Stromeyer.)](https://iiif.wellcomecollection.org/image/b21713613_0472.jp2/full/800%2C/0/default.jpg)