A dictionary of chemical solubilities : inorganic / by Arthur Messinger Comey.
- Comey Arthur Messinger, 1861-
- Date:
- 1896
Licence: Public Domain Mark
Credit: A dictionary of chemical solubilities : inorganic / by Arthur Messinger Comey. Source: Wellcome Collection.
Provider: This material has been provided by the Royal College of Physicians of Edinburgh. The original may be consulted at the Royal College of Physicians of Edinburgh.
475/544 (page 451)
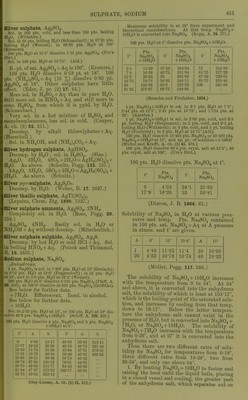
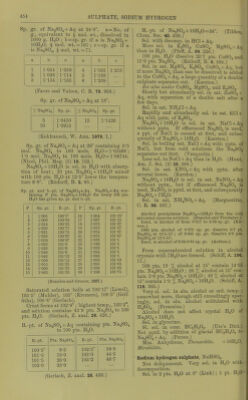
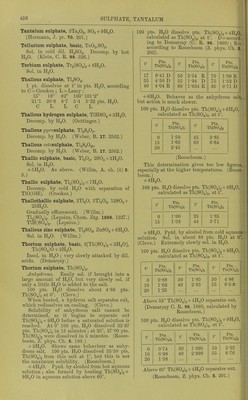
![Silver sulphate, AgjSO.. Sill, in JdO pts. cold, and less than 100 pts. boiling n.iO. (Wittstein.) Sol. in SS pts. boiling H..O (Schnaub.irt); in S7-25 pts. boiling HoO (Wenzel); iu ()8-S5 pts. H.jO at 100 (KrenuTsX , 100 pts. H..O at IS-S dissolve 1-15 pts. Ag.jSO^. (Ures Diet.) Sol. in 160 pts. H2O at 18-75°. (Abl.) B.-i>t. of sat. Ag.,S04 +Aq is 100°. (Kremers.) 100 pts. H2O dissolve 0-58 pt. at 18°. 100 pts. (NH4)oS04 + Aq (15 %) dissolve 0-85 pt. AggSO^ lit 18°. Other sulphates liave little eflect. (Eder, J. pr. (2) 17. 44.) More sol. in HoSO^ + Aq than in pure HjO. Still more sol. in'HNOg + Aq and still more in cone. HoSOj, from which it is pptd. by HgO. (Schnaubart.) Very sol. in a hot mixture of H0SO4 and monobrombenzene, less sol. in cold. (Couper, A. ch. (3) 62. 311.) Decomp. by alkali thiosulphates +Aq. (Herschell.) Sol. in NH4OH, and CNH4)oC03 + Aq. Silver hydrogen sulphate, AgHSOj. Decomp. by HoO ; sol. in H.^SOj. (Slas.) Ag.,0, 3H„0,'' 4S03 + 2H20 = AgH3{S04)2 + HoO. As above. (Schultz, Pogg. 133. 137.) 2Ag20, 3H2O, 5S03 + 2H20 = Ag4H8(S04)5 + 2H2O. As above. (Schultz.) Silver 7)?/rosulphate, AgoSjO?. Decomp. by HoO. (Weber, B. 17. 2497.) Silver thallic sulphate, AgTl(S04)2. (Lepsius, Chem. Ztg. 1890. 1327.) Silver sulphate ammonia, Ag2S04, 2NH3. Completely sol. in H,0. (Rose, Pogg. 20. 153.) Ag._,S04, 4NH3. Easily sol. in HgO or NH4OH + Aq without decomp. (Mitscherlich.) Silver sulphate sulphide, Ag2S04, AgjS. Decomp. by hot H.,0 or cold HCl + Aq. Sol. in boiling HN03 + Aq. (Poleck and Thiimmel, B. 16. 2435.) Sodium sulphate, Na2S04. Aiihydrous. 1 pt. Na2S04 is sol. in 7-367 pts. H2O at 15° (Gerlach); in 8-52 pts. H2O atia-a (Poggendorf); in 10 pts. H.)0 at 13°, and in 3-3 pts. H..0 at 02-2° (Wenzel). 100 pts. H2O at 0' dissolve 5-155 pts. Na.,H04 (PfalT, A. 99. 220); at 100-0° dissolve 45-985 pts. Na2S04 (Griffiths). See below for further data. + 7H2O. Efflorescent. Insol. in alcohol. See below for further data. + IOH2O. Sol. in 2-33 pts. H2O at 19°, or 100 pts. H.,0 at 19' dis- solve 42-8 pts. Na2SO4-)-10H2O. (Schiff, A.' 109. 320.) 100 pts. H2O dissolve a pts. Na.;S04 and b pts. Na2S04 + IOH2O at t°. _ a b t a b 0 5-02 12-17 83-88 50-04 312-11 11-67 10-12 26-88 40-15 48-78 291-44 18-30 11-74 31-38 45-04 47-81 276-91 17-91 16-73 48-28 50-40 40-82 262-35 25-05 28-11 99-48 59-79 46-42 28-76 87-85 101-53 70-61 44-35 30-75 48-05 215-77 84-42 42-96 31-84 47-37 270-22 103-17 42-66 8273 50-65 322-12 (Gay-Lussac, A. ch. (2) H, 812.) Maximum solubility Is at 83° from experiment and theoretical considerations. At this temp. NnoH04+ lOH.jO is converted into Na2S04. (Kopp, A. 34. 271.) 100 pts. HjO at t° dissolve pts. Na2SO4+10H2O. t° Pts. Na2S04 +IOH2O t° Pts. Na2804 +IOH2O 1 1 Pts. Na.^J04 + IOH2O 2-5 11-39 37-50 •294-04 75 241-68 7-5 16-38 43-75 201-04 81-25 217-20 12-5 29-03 50 285-06 87-50 220-65 18-75 70-78 56-25 248-11 i 93-75 225-46 25 143-38 62-5 222-22 100 241-69 31-25 479-97 68-75 242-88 (Brandes and Flrnhaber, 1824.) 1 pt. Na.>SO4+10HoO is sol. in 6-1 pts. H2O at 7-5°; 3-44 pts. at 12-5°; 2-41 pts. at 18-76°; and 1-724 pts. at 20°. (Karsten.) 1 pt. Na.HS04-f IOH2O is sol. in 2-86 pts. cold, and 0-8 Et. boiling H.iO (Borgmann); in 3 pts. cold, and 0-5 pt. oiling H.)0 (Wittstein); in 4 pts. cold, and 1 pt. boiling H..0 (Fourcroy); in 3 pt.s. H.,0 at 18-75° (Abl). 100 pts. H2O dissolve 12-494 pts. NaaSOj or 35-492 pts. NaoSO4+10H2Oat 15°, and sp. gr. of solution = 1-10847 (Michel and Kraflt, A. ch. (3) 41. 478.) 100 pts. H2O dissolve 39-4 pts. cryst. salt at 16-6°; 80 pts. cryst. salt at 100°. (Ure s Diet.) 100 pts. HgO dissolve pts. ]Sra2S04 at t. t° Pts. Na2Sb4 t° Pts. Na2S04 0 17-9 4-53 16-28 24-1 33 25-92 50-81 (Diacon, J. B. 1866. 61.) Solubility of Na2S04 in H2O at various pres- sures and temp. Pts. Na2S04 contained in 100 pts. sat. Na5S04 + Aq at A jiressnre in atmos. and t° are given. A 0° 15° 15-4° A 15 1 20 4-40 4-53 11-32 10-78 11-4 10-74 30 40 10-05 10-33 (Mbller, Pogg. 117. 386.) The solubility of Na^SO4 + 10H2O increases with the temperature from 0 to 34°. At 34° and above, it is converted into the anhydrous salt, the solubility of which is least at 103-17°, which is the boiling-point of the saturated solu- tion, and increases by cooling from that temp, down to 18-17°. Below the latter tempera- ture the anhydrous salt cannot e.xist in the presence of H„0, but is converted into Na.,S04-f 7H2O, or Na.2SO4-t-10H2O. The solubility of Na^S04 + 7H20 increases with the temperature from 0-26°, and at 27° it is converted into the anhydrous salt. Thus there are two different rates of solu- bility for Na.^S04 for temperatures from 0-18°, tliree dillercnt rates from 18-26^ two from 26-34°, and only one above 34°. 1. By heating Na-^SOj-flOHp to fusion and raising the heat until the li(iuid boils, placing in a closed vessel and cooling, the greater part of the anhydrous salt, which separates out on](https://iiif.wellcomecollection.org/image/b21713613_0475.jp2/full/800%2C/0/default.jpg)