A dictionary of chemical solubilities : inorganic / by Arthur Messinger Comey.
- Comey Arthur Messinger, 1861-
- Date:
- 1896
Licence: Public Domain Mark
Credit: A dictionary of chemical solubilities : inorganic / by Arthur Messinger Comey. Source: Wellcome Collection.
Provider: This material has been provided by the Royal College of Physicians of Edinburgh. The original may be consulted at the Royal College of Physicians of Edinburgh.
476/544 (page 452)
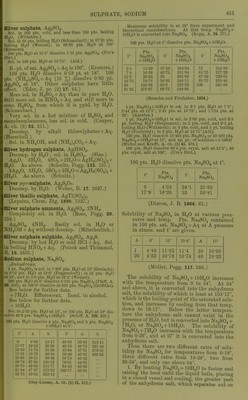
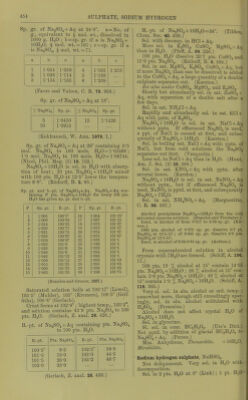
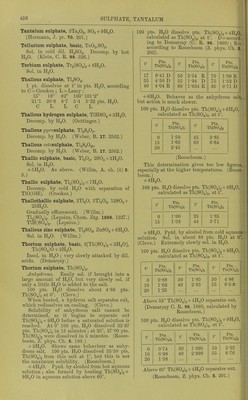
![heating, redissolves on cooling, and the amount increases as the temp, falls until 18° is reached. Below 18° NaaSO^ + 7 H2O is formed. Saturated Na.jS04 + Aq thus obtained contains for 100 pts. H 0 at: 18° 20° 53-25 52-76 30° 33° 50-37 49-71 25° 26° 51-53 51-31 pts. Na2S04, 34° 36° 49-53 49-27 pts. Na2S04. 2. By allowing the boiling saturated solution free from undissolved salt to cool to 0° with ex- clusion of air until crystals of NagSOj + 7H2O are formed, then removing the greater part of the mother liquor with a warm pipette, and warming the rest of the mother liquor with the excess of crystals, the crystals dis- solve in increasing quantity between 0° and 26-27°, so that at 27° the solution contains 56 pts. NajSOi to 100 pts. H2O. The remain- ing undissolved crystals of Na2S04 -t- 7H2O begin to melt very slowly at 27°, more quickly at higher temperatures, and cause the separation of anhydrous crusts, and thus the strength of the solution is gradually lowered to the normal. Saturated solutions prepared in this way con- tain for 100 pts. H2O at: 0° 10° 13° 19-62 30-49 34-27 pts. Na2S04, or 44-89 78-9 92-9 pts. Na2S04-t-7H20, 15° 16° 17° 37-48 38-73 39-99 pts. Na2S04, or 105-8 117-4 111-0 pts. Na2S04-l-7H20, 18° 19° 20° 41-63 43-35 44-73 pts. Na2S04, or 124-6 133-0 140-0 pts. Na2S04-t-7H20, 25° 26° 52-94 54-97 pt s. Na2S04. or 188-5 202-6 pt s. Na2S04 + 7H20, 3. Solutions obtained by shaking H2O with Na2SO4-l-10H2O contain for 100 pts. HgO at: 15° 13-20 pts. Na2S04, 35-96 pts. Na2SO4-H0H2O, 25° 28-00 pts. ]Sra2S04, 98-48 pts. NaoS04-(-10H20, 0° 10° 5-02 9-00 or 12-16 23-04 18° 20° 16-80 19-40 or 48-41 58-85 26° 30° 30-00 40-00 pts. NaoSOj, or 109-81 184-1 pts. Na2SO4-fl0H2O, 33° 34° 50-76 55-0 pts. ]Sra2S04. or 323-1 412-2 pts. Na2S04-f IOH2O. At 34° Na2S04-MOH20 begins to melt in its crystal H2O. As long as there is a considerable quantity of unchanged crystals present, the solution contains 55 pts. NagSOj for 100 pts. H2O, but as the hydrous salt decreases in amount and becomes converted into the an- hydrous salt, the solution becomes weaker and contains only 49-53 pts. NaaSOj for 100 pts. H2O after M'/irming for 6 or 8 hours at 34°. In the same way tem])orary solutions can be ob- tained at 36-40° witli 55-56 pts. Na^SOj to 100 pts. H2O, but tins amount sinks to the normal even more quickly tlian at 34°. Na2S04 dehydrated at 100-150°, after the addition of 1|-1§ ])ts. H2O, gives a solution be- tween 0° and 32° of tiie same strength as NagSOj-l-lOHaO, but at 34° a solution with 55 pts. Na2S04 to 100 pts. H2O cannot be ob- tained, but one with 49-53 pts. is formed (Lbwel, A. ch. (3) 49. 32.) 4. Solubility of anhydrous salt. Above 34°, 100 pts. HoO dissolve at: 35° 40° 45° 50° 55° 50-2 48-8 47-7 46-7 45-9 pts. Na2S04, 60° 65° 70° 75° 80° 45-3 44-8 44-4 44-0 43-7 pts. Na2S04, 85° 90° 95° 100° 103-5° 43-3 43-1 42-8 42-5 42-2 pts. Na2S04, (Mulder.) Solubility in 100 pts. H2O at t° t° IT La. t° t° Pf-G Na2S04 Na2S04 NaoSOi 0 4-8 35 50-2 70 44-4 1 5-1 36 49-9 71 44-3 2 5-4 37 49-6 72 44-2 3 5-7 38 49-3 73 44-2 4 6-0 39 49-1 74 44-1 5 6-4 40 48-8 75 44-0 6 6-8 41 48-5 76 44-0 7 7-3 42 48-3 77 43-9 8 7-8 43 48-1 78 43-8 9 8-4 44 47-9 79 43-7 10 9-0 45 47-7 80 43-7 11 9-7 46 47-5 81 43-6 12 10-5 47 47-3 82 43-5 13 11-4 48 47-1 83 43-5 14 12-4 49 46-9 84 43-4 15 13-4 50 46-7 85 43-3 16 14-5 51 46-6 86 43-3 17 15-7 52 46-4 87 43-2 18 16-9 53 46-2 88 43-2 19 18-2 54 46-1 89 43-1 20 19-5 55 45-9 90 43-1 21 20-9 56 45-8 91 43-0 22 22-5 57 45-7 92 43-0 . 23 24-1 58 45-6 93 42-9 ' 24 25-9 59 45-4 94 42-9 25 27-9 60 45-3 95 42-8 26 30-1 61 45-2 96 42-7 27 32-4 62 45-1 97 42-6 28 35-0 63 45-0 98 42-6 29 37-8 64 44-9 99 42-5 30 40-9 65 44-8 100 42-5 31 44-2 66 44-7 101 42-4 32 47-8 67 44-6 102 42-3 32-75 50-65 68 44-5 103 42-2 33 50-6 69 44-5 103-5 42-2 34 50-4 ... (Mulder, Scheik. Verhandel. 1864. 123.)](https://iiif.wellcomecollection.org/image/b21713613_0476.jp2/full/800%2C/0/default.jpg)