A dictionary of chemical solubilities : inorganic / by Arthur Messinger Comey.
- Comey Arthur Messinger, 1861-
- Date:
- 1896
Licence: Public Domain Mark
Credit: A dictionary of chemical solubilities : inorganic / by Arthur Messinger Comey. Source: Wellcome Collection.
Provider: This material has been provided by the Royal College of Physicians of Edinburgh. The original may be consulted at the Royal College of Physicians of Edinburgh.
478/544 (page 454)
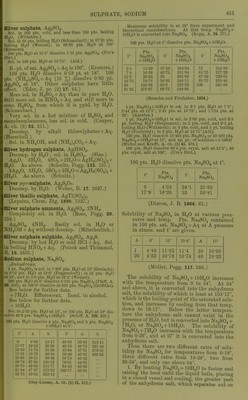
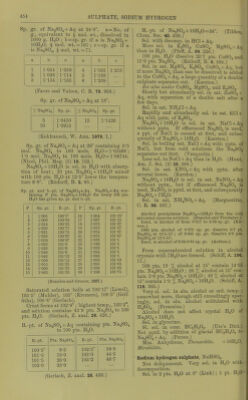
![Sp. gr. of Na„SOj + Aq at 24-8°. a = No. of g., equivalent to ^ uiol. wt., dissolved in 1000 g. HaO ; b = sp. gr. if a is NaoS04 + lOHgO, i mol. wt. =161 ; c=:si). gr. if a is NajSO^, I mol. wt. =71. a b G a b c 1 1-054 1-059 4 1-163 1-213 2 1-098 1-114 5 1-188 3 1-134 1-165 6 1-209 (Favre and Valson, C. R. 79. 968.) Sp. gr. of Na2S04 + Aq at 18°. X Na2S04 Sp. gr. % Na2S04 Sp. gr. 5 10 1-0450 1-0915 15 1-1426 (Kohkausch, W. Ann. 1879. 1.) Sp. gr. of NaoS04 + Aq at 20° containing 0-5 mol. ]Sra2S04 to 100 mols. HgO = 1-03466 ; 1-0 mol. Na2S04 to 100 mols. HaO^ 1-06744. (Nicol, Phil. Mag. (5) 16. 122.) Na2SO4 + 10H2O is sol. in HgO with absorp- tion of heat; 20 pts. Na2S04 +lOHjO mixed with 100 pts. HgO at 12-5° lower the tempera- ture 6-8°. (Riidorff, B. 2, 68.) Sp. gr. and b.-pt. of Na2S04-(-Aq. Na2S04-fAq con- taining P pts. Na2SO4-M0H2O for every 100 pts. H2O has given sp. gr. and b.-pt. p Sp. gr. B.-pt. P Sp. gr. B.-pt. 1 1-005 100-5° 16 1-064 101-25° 2 1-008 100-62 17 1-067 101-25 3 1-014 100-62 18 1-070 101-37 4 1-020 100-75 19 1-072 101-37 5 1-021 100-75 20 1-074 101-37 6 1-028 100-87 21 1-076 101-37 7 1-030 100-87 22 1-078 101-5 8 1-032 loro 23 1-080 101-5 9 1-030 101-0 24 1-082 101-5 10 1-040 101-0 25 1-084 101-5 11 1-043 101-12 26 1-090 101-5 12 1-050 101-12 27 1-092 101-63 13 1-055 101-25 28 1-095 101-63 14 1-060 101-25 29 1-098 101-63 15 1-062 101-25 30 1-100 101-75 (Brandes and Gruner, 1827.) Saturated solution boils at 103-17° (Lowel), 103-5° (Mulder), 105° (Kremers), 100-5° (Grif- fiths), 100-8° (Gerlach). Crust forms at 102-9°; highest temp., 103-2 , and solution contains 43-9 pts. Na2S04 to 100 pts. H2O. (Gerlach, Z. anal. 26. 426.) B.-pt. of Na2S04-»-Aq containing pts. Na2S04 to 100 pts. H2O. B.-pt. Pts. Na2S04 B.-pt. Pts. Na2S04 100-5° 9-5 102-5° 39-0 101-0 18-0 103-0 44-5 101-5 26-0 103-2 46-7 102-0 33-0 (Gerlach, Z. anal. 26. 430.) M.-pt. of Na2SO4-|-10H2O = 34°. (Tilden, Chem. Soc. 46. 409.) Sol. with docomp. in HCl-l-Aq. More sol. in K2SO4, GUSO4, MgSO. + Aq than in H2O. (PfaH', A. 99. 226.) 100 pts. H2O dissolve 20-7 pts. CuSO. and 15-9 pts. Na2S04. (Rudorff, B. 6. 484.) Sol. in sat. MgS04, K2SO4, CuS04-fAq, but if more Na2S04 than can be dissolved is added to the GuS04-t-Aq, a large quantity of a double sulphate separates out. (Karsten.) See also under GUSO4, MgS04, and K2SO4. Slowly but abundantly sol. in sat. ZnS04 + Aq, with separation of a double salt after a few days. SoL in sat. NHjCl-t-Aq. Rapidly and abundantly sol. in sat. KCl-f Aq with pptn. of K2SO4. Na2S04-f IOH2O is sol. in sat. NaCl-t-Aq without pptn. If effloresced Na2S04 is used, a p2)t. of NaCl is caused at first, and subse- quently of Na2S04 + IOH2O. (Karsten.) Sol. in boiling sat. NaCl+Aq with pptn. of NaCl, but from cold solutions the Na2S04 separates out first. (Vauquelin.) Less sol. in NaCl-F Aq than in H2O. (Hunt, Am. J. Sci. (2) 25. 368.) Sol. in sat. KNOg-f Aq with pptn. after several hours. (Karsten.) Na2SO4-H0H2O is sol. in sat. NaNOg-t-Aq without pptn., but if effloresced Na2S04 is used, NaNOg is pptd. at first, and subsequently Na2S04-f7H20. Sol. in sat. NH4N03-J-Aq. (Margueritte, C. R. 38. 307.) Alcohol precipitates Na2SO4-f-10H2O from the cold saturated aqueous solution. (Brandes and Firnhaber.) Insol. in alcohol of from 0-817 to 0-90 sp. gr. (Kir- wan.) 1000 pts. alcohol of 0-872 sp. gr. dissolve 0-7 pt. Na^S04 at 12-5-15°; of 0-905 sp. gr. dissolve 3-8 pts. NaoS04 at 12-5-15°. Insol. in alcohol of 0-83-0-85 sp. gr. (Anthon.) From supersaturated solution in alcohol crystals with 7H2O are formed. (Schiff, A. 106. 11.) 100 pts. 10 % alcohol at 15° contain 14-35 pts. Na2S04-f lOHoO ; 20 % alcohol at 15° con- tain 5-6 pts. Na2S04-f IOH2O ; 40 % alcohol at 15° contain 1 -3 % Na2S04 + IOH2O. (Schiff, A. 118. 365.) Very si. sol. in abs. alcohol at ord. temi). ; somewhat more, though still exceedingly spar- ingly, sol. in abs. alcohol acidulated with H2SO4. (Fresenius.) Alcohol does not affect crystal HjO of Na2S04-f IOH2O. Sol. in glycerine. SI. sol. in cone. HC2H3O2. (Ure's Diet.) Not pptd. by addition of glacial HCjHgOo to Na2S04-fAq. (Persoz.) Min. Anhydrous, ThenardUc. -t-lOHgO, Mirabilite. Sodium hydrogen sulphate, NaHS04. Not deliquescent. Very sol. in HjO with decom])osition. Sol. in 2 pts. H2O at 0° (Link); 1 pt. HoO ■](https://iiif.wellcomecollection.org/image/b21713613_0478.jp2/full/800%2C/0/default.jpg)