A dictionary of chemical solubilities : inorganic / by Arthur Messinger Comey.
- Comey Arthur Messinger, 1861-
- Date:
- 1896
Licence: Public Domain Mark
Credit: A dictionary of chemical solubilities : inorganic / by Arthur Messinger Comey. Source: Wellcome Collection.
Provider: This material has been provided by the Royal College of Physicians of Edinburgh. The original may be consulted at the Royal College of Physicians of Edinburgh.
480/544 (page 456)
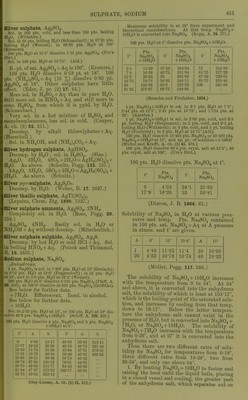
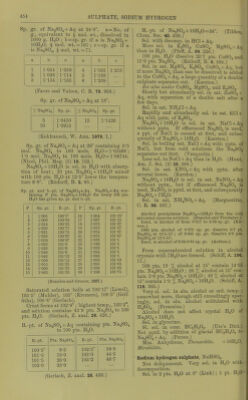
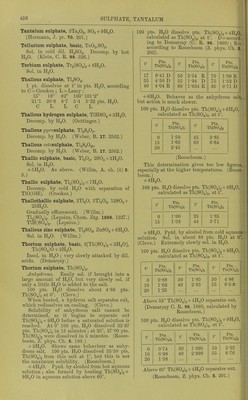
![Tantalum sulphate, 3Ta.j05, SO^ + 9HaO. (ircriiuum, J. pr. 70. 201.) Tellurium sulphate, basic, TeOa.SOg. Sol. in cold dil. H2SO4. Decomp. by hot H2O. (Klein, C. R. 99. 326.) Tferbium sulphate, Ti2(S04)., + 8H„0. Sol. in HoO. Thallous sulphate, TlgSO^. 1 pt. dissolves at t° in pts. HjO, according to C = Crookes ; L = Lamy : 15° 18° 62° 100° 101-2° 21-1 20-8 8-7 5-4 5-22 pts. HoO. C L L C L Thallous hydrogen sulphate, TIHSO4 + 3H2O. Decomp. by HoO. (Oettinger.) Thallous ^J2/''osulphate, TI2S2O7. Decomp. by H2O. (Weber, B. 17. 2502.) Thallous ociosulphate, TlgSgOas. Decomp. by H2O. (Weber, B. 17. 2502.) Thallic sulphate, basic, TlgOg, 2SO3 + 3H2O. Sol. in H2O. + 5HoO. As above. (Willm, A. oh. (4) 5. 5.) Thallic sulphate, Tl2(S04)3 + 7H20. Decomp. by cold HgO with separation of TIO(OH). (Crookes.) Thallothallic sulphate, 2TI2O, STlgOg, I2SO3 + 25H2O. Gradually efflorescent. (Willm.) Tl2(S04)2. (Lepsius, Chem. Ztg. 1890. 1327.) T1H(S04)2. (Lepsius.) Thallous zinc sulphate, TI2SO4, ZnS04 + 6H20. Sol. inH20. (Wilbn.) Thorium sulphate, basic, 3[Th(S04)2 + 2H20], Th(S04)0 + 2H20. Insol. in HgO ; very slowly attacked by dil. acids. (Demar9ay.) Thorium sulphate, Th(S04)„. Anhydrous. Easily sol. if brought into a large amount of H2O, but very slowly sol. if only a little H2O is added to the salt. 100 pts. H2O dissolve about 4'86 pts. Th(S04)2 at 0°. (Cleve.) When heated, a hydrous salt se^^arates out, which redissolves on cooling. (Cleve.) Solubility of anhydrous salt cannot be determined, as it begins to separate out Th(S04)2 +9H2O before a saturated solutionis reached. At 0° 100 pts. HoO dissolved 22-97 pts. Th(S04)2 in 15 minutes; at 25°, 27-00 pts. Th(S04)2 were dissolved in 5 minutes. (Rooze- boom, Z. phys. Ch. 5. 198.) + 2H2O. Shows same behaviour as anhy- drous salt. 100 pts. HoO dissolved 35-50 pts. Th(S04)2 from this salt at 1°, but this is not the maximum solubility. (Roozeboom.) + 4H2O. Pptd. by alcohol from hot aqueous solution; also formed by heating Th(S04)2 + 9H0O in aqueous solution above 60°. 100 pts. H2O dissolve pts. Th(S04)2 + 4H,0, calculated as Th(S04)o, at t°. D = accord- ing to Demar9ay (C. R. 96. 1860) ; R= according to Roozeboom (Z. phys. Ch. 6 202). t° Pts T11(S64)2 t° Pts Th(864>2 t Pts. Th(S04>2 17 9-41 D 50 2-54 R 70 1-09 R 35 4-50 D 55 1-94 D 75 1-32 D 40 4-04 R 60 1-634 R 95 0-71 D + 6H2O. Behaves as the anhydrous salt, but action is much slower. 100 pts. H2O dissolve pts. Th(S04)2 + 6H20, calculated as Th(S04)2, at t°. t° Pts. Th(s64>2 t Pts 111(864)2 0 1-50 45 3-85 15 1-63 60 6-64 30 2-45 (Roozeboom.) This determination gives too low figures, especially at the higher temperatures. (Rooze- boom.) -f 8H2O. 100 pts. H2O dissolve pts. Th(S04)2 4-8H2O, calculated as Th(S04)2, at t°. t° Pts Th(s64)2 t° Pts T1I(S64)2 0 15 1-00 1-38 25 44 1-85 3-71 + 9H2O. Pptd. by alcohol from cold aqueous solution. Sol. in about 88 pts. H2O at 0°. (Cleve.) Extremely slowly sol. in HgO. 100 pts. H2O dissolve pts. Th(S04)2 + 9H2O, calculated as Th(S04)2, at t°. t° Pts. 111(864)2 r Pts. Th(s64)2 t Pts T1.(S64>, 0 0-88 30 1-85 50 4-86 10 1-02 40 2-83 55 6-o± 20 1-25 Above 55° Th(S04)2 + 4H2O separates out. (Demar9ay C. R. 96. 1860, calculated by Roozeboom.) 100 pts. H2O dissolve pts. Th(S04)2 +9HoO, calculated as Th(S04)„, at t°. t° Pts. T11(S64)2 t° Pts. Tll(S04>2 t° Pts. Tll(S04>2 0 0-74 30 1-995 50 5-22 10 0-98 2-998 56 6-76 20 1-38 1 ... Above 60° Th(S04)2 + 4H20 separates out. (Roozeboom, Z. phys. Ch. 6. 201.)](https://iiif.wellcomecollection.org/image/b21713613_0480.jp2/full/800%2C/0/default.jpg)