A dictionary of chemical solubilities : inorganic / by Arthur Messinger Comey.
- Comey Arthur Messinger, 1861-
- Date:
- 1896
Licence: Public Domain Mark
Credit: A dictionary of chemical solubilities : inorganic / by Arthur Messinger Comey. Source: Wellcome Collection.
Provider: This material has been provided by the Royal College of Physicians of Edinburgh. The original may be consulted at the Royal College of Physicians of Edinburgh.
68/544 (page 44)
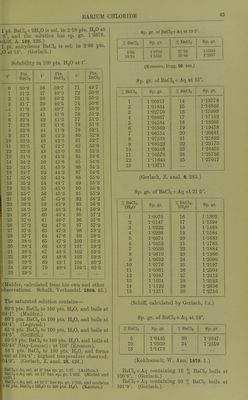
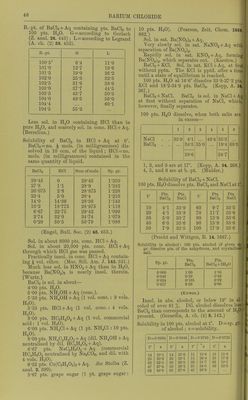
![AURICYANHYDRIC ACID Auricyanhydric acid, HAu(CN)^ + i^H20. Easily sol. in H2O, alcohol, or ether. See also Bromauricyanides. Chlorauricyanides. lodauricyanides. Ammonium auricyanide, NH4Au(CN)4. Easily sol. in H«0 or alcohol. Insol. in ether. Cobaltous auricyanide, Co[Au(CN)4]2 + 9H20. SI. sol. in cold, easily in hot H2O. SI. sol. in alcohol. (Lindbom.) Potassium auricyanide, KAu(CN)4 + lJH20. ElHorescent. SI. sol. in cold, easily in hot H2O. Easily sol. in alcohol. Silver auricyanide, AgAuCN4. Insol. in HoO or HNOj + Aq. Sol. in NH40H + Aq. iJiaurofZzamine nitrate. Sec Auriimide nitrate. Aurobromhiydric acid. See Bromauric acid. Aurobromic acid. Sc; Bromauric acid. Aurochlorhydric acid. See Chlorauric acid. Aurochloric acid. Sec Chlorauric acid. Azoimide, HNj. Miscible with HjO and alcohol. (Curtius and Radershausen, J. pr. (2) 43. 207.) For salts of HN3, see azoimide of metal under metal. Azophosphoric acid. See Pi/' ophosphamic acid. ZJewiazophosphoric acid. See P;/; ophospho(^iamic acid. Barium, Ba. Decomposes HgO at ordinary temperature. Barium azoimide, BaNg. Easily sol. in HjO. (Curtius, B. 23. 3023.) Barium bromide, BaBr2 + 2E[20. 100 pts. H.,0 dissolve— atO° 20° 40° 60° 80° 100° 98 104 114 123 135 149 yts. BaBrg. Sat. BaBrj + Aq boils at 113°. (Ki-emers, Pogg. 99. 43.) Sp. gi-. of BaBr2 +Aq at 19 5° containing : 5 10 15 20 25 30 % BaBrg, 1-045 1-092 1-114 1-201 1-262 1-329 35 40 45 50 55 % BaBra. 1-405 1-485 1-580 1-685 1-800 (Kremers, Pogg. 99. 444, calculated by Gerlach, Z. anal. 8. 285.) Very sol. in absolute alcohol. (Hiinefeld.) 100 pts. absolute methyl alcohol dissolve 50 pts. BaBrgat 22-5°. 100 pts. absolute ethyl alcohol dissolve ,3 pts. BaBrj at 22-5°. (do Bruyn, Z. phys. Ch. 10. 783.) Sat. solution in 87 % alcohol contains 6 % BaBrg. (Richards, Z. anorg. 3. 455.) 100 pts. absolute methyl alcohol dissolve 45-8 pts. BaBr2 + 2H20 at 15°. 100 pts. 93-5 % methyl alcohol dissolve 27-3 pts. BaBr2 + 2H20 at 15°. 100 i)ts. 50 % methyl alcohol dissolve 4 pts. BaBr„ + 2H20 at 15°. (de Bruyn, Z. phys. Ch. 10. 787.) Nearly insol. in boiling amyl alcohol, 10 ccm. dissolving only an arat. equal to 1*3 mg. BaO. (Browning, Sill. Am. J. 144. 459.) Bariimi cadmium bromide, BslBt^, CdBro + 4H2O. Sol. in H2O. (v. Hauer, W. A. B. 20. 40.) Barium carbide, BaC2. Decomp. by HoO. (Maquenne, C. R. 144. 360.) Bariimi chloride, BaClj, and + 2H2O. Permanent in dry air. 100 pts. HoO at t° dissolve (a) pts. BaClo and (6) pts. BaClo+2H20. t° a 6 t° a b 15-64 84-86 43-50 74-89 59-94 65-51 49-31 43-84 55-63 105-48 59-58 77-89 (Gay-Lussac, A. ch. (2) 11. 309.) 100 pts. H2O at t° dissolvo 32-G2+0-2711t pts. BaCl». (Kopp.) 100 pts. H2O dissolve pts. BaCl2+2H20 at t°. f Pts. BaCl2+2H.20 t Pts. BaCl2+2H20 16-25 89-66 62-50 48-0 20-00 42-22 75-00 63-0 22-50 43-7 87-00 65-0 37-50 51 0 100 72-0 50-00 65-0 (Brandes.) Sol. in 2-67 pts. HoO at 18-75°. (Abl.) 1 pt. BaClo is sol. in 2-86 pts. H2O at 15-5°, and 1*67 pts. at boiling temp. (M. R. and P.) 100 pts. HoO at 15-5° dissolve 20 pts. BaCl2, and 43 pts. at 87-7°. (Ure's Diet.) Solubility in 100 pts. H2O at t°. t° Pts. BaCl2 t° Pts. BaCl2 0 12-2 38-4 62-75 31-1 33-9 41-2 47-7 77-5 95-65 102-5 105 51-9 57- 7 58- 9 59- 7 (Nordonskiokl, Pogg. 136. 316.) 100 pts. H2O dissolve pts. BaOIg at t°. t° Pts. BaCl2 t° Pts. BaClo 9 30 87 83-2 38-1 40 0 50 58 48-7 45-!i (Gerardin, A. ch. (4) 6.143.) 1](https://iiif.wellcomecollection.org/image/b21713613_0068.jp2/full/800%2C/0/default.jpg)