A dictionary of chemical solubilities : inorganic / by Arthur Messinger Comey.
- Comey Arthur Messinger, 1861-
- Date:
- 1896
Licence: Public Domain Mark
Credit: A dictionary of chemical solubilities : inorganic / by Arthur Messinger Comey. Source: Wellcome Collection.
Provider: This material has been provided by the Royal College of Physicians of Edinburgh. The original may be consulted at the Royal College of Physicians of Edinburgh.
70/544 (page 46)
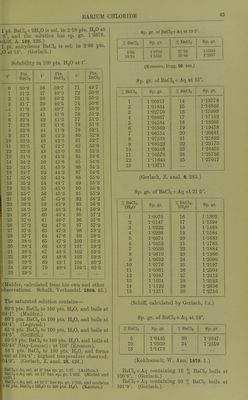
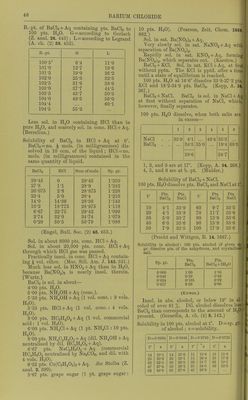
![B.-pt. of BaCla + Aq containing pts. BaCla to 100 pts. H„0. G = according to Gerlacli (Z. anal. 26. 443); L=according to Legrand (A. ch. (2) 69. 452). 13. -pL. d L 100-5° 6-4 11-0 101-0 12-7 19-6 101-5 19-0 26-2 102-0 25-3 32-5 102-5 31-6 38-6 103-0 37-7 44-5 103-5 43-7 50-3 104-0 49-5 56-0 104-4 60-1 104-5 55-2 Less sol. in HoO containing HCl than in pure HgO, and scarcely sol. in cone. HCl + Aq. (Berzelius.) Solubility of BaClj in HCl + Aq at 0°. BaC]2 = no. ^ mols. (in milligi-ammes) dis- solved in 10 ccni. of the liquid ; HCl = no. mols. (in milligrammes) contained in the same quantity of liquid. HCl Sum of mols. Sp. gr. 29-45 0 29-45 1-250 27-8 1-1 28-9 1-242 26-075 2-8 28-875 1-228 23-4 5-0 28-4 1-210 14-0 14-36 28-36 1-143 10-2 18-775 28-975 1-118 6-67 22-75 29-42 1-099 2-74 32-0 34-74 1-079 0-29 50-5 50-79 1-088 (Engel, Bull. Soc. (2) 45. 653.) Sol. in about 8000 pts. cone. HCl + Aq. Sol. in about 20,000 pts. cone. HCI + Aq through which HCl gas was passed. Practically insol. in cone. HCl + Aq contain- ing J vol. ether. (Mar, Sill. Am. J. 143. 521.) Much less sol. in HNOg-t-Aq than in H2O, because Ba(N03)2 is nearly insol. therein. (Wurtz.) BaClo is sol. in about— 4- 00 pts. HjO. 5 -00 pts. NH4OH + Aq (cone.). 5- 33 pts. NH^OH-I-Aq (1 vol. cone.: 3 vols. H2O). 5- 33 pts. HCl-fAq (1 vol. cone: 4 vols. HjO). 8-00 pts. HCaHgOa-l-Aq (1 vol. commercial acid: 1 vol. H2O). 6- 00 pts. NH4Cl-l-Aq (1 pt. NH^Cl: 10 pts. H5O). 6-00 pts. NH^HsOo-f Aq (dil. NH40H-1-Aq neutralised by dil. HCaHaOa + Aq). 6-67 pts. NaCaHsOo 4-Aq (commercial HCoHsOa neutralised by NaaCOs, and dil. with 4 vols. H2O). 6-33 pts. Cu(C2H302)a + Aq. See Stolba (Z. anal. 2. 390). 5-67 pts. grape sugar (1 pt. grape sugar: 10 pts. H2O). (Pearson, Zeit. Chem. 1869. 662.) Sol. in sat. Ba(N03)2-f Aq. Very slowly sol. in sat. NaNOj-f-Aq with separation of Ba(N03)2. Rapidly sol. in sat. KNOg + Aq, forming Ba(N03)2, which separates out. (Karsten.) BaClo-hKCl. Sol. in sat. KCl-hAq, at first without pptn. The KCl is pptd. after a tiiii^ until a state of equilibrium is reached. 100 pts. H2O at 16-6° dissolve 33-8-27-2 pts. KCl and 18-2-34-9 pts. BaCl2. (Kopp, A. 31 267.) BaCl2 + NaCl. BaCla is sol. in NaCl-f Aq at first without separation of NaCl, which, however, finally separates. 100 pts. HjO dissolve, when both salts are in excess— 1 2 3 4 5 r, NaCl . . 35-9 4-1 40-4 35-3 BaClo . . 34-5 35-0 19-4 60-3 38-6 54-7 1, 2, and 3 are at 17°. (Kopp, A. 34. 268.) 4, 5, and 6 are at b.-pt. (Mulder.) Solubility of BaCla-fNaCl. 100 pts. H2O dissolve pts. BaClg and NaCl at Pta. BaCl2 Pts. NaCl t° Pts. BaCl2 Pts. NaCl 10 4-1 33-9 60 9-7 33-5 20 4-1 33-8 70 11-7 33-6 30 5-0 33-7 80 13-9 33-6 40 6-3 33-6 90 15-9 33-6 50 7-9 33-5 100 17-9 33-6 (Precht and Wittgen, B. 14. 1667.) Solubility in alcohol: 100 pts. alcohol of given sji gr. dissolve pts. of the anhydrous,. and crystalliseii salt. Sp. gr. Pts. BaCl2 Pts. BaCl2-|-2H20 0-900 1-00 1-56 0-848 0-29 0-43 0-834 0-185 0-32 0-817 0-09 0-06 (Kirwan.) Insol. in abs. alcohol, or below 19° in '1 cohol of over 91 %. Dil. alcohol dissolve ^ BaCla than corresponds to the amount of II .'' present. (Gerardin, A. ch. (4) 6, 142.) Solubility in 100 pts. alcohol at t°. D = s] of alcohol; s — solubility. D = 0-9904 D = 0-9848 D = 0-9793 D = 0-9726 t° s t° s t • « s t° s 14 29-1 14 25-0 11 19-6 15 15-6 25 32-0 32 29-1 15 20-4 23 17-0 32 33-5 39 30-9 20 21-7 33 19-1 47 37-4 50 33-2 35 24-6 50 22-0 60 39-8 63 37-6 45 26-8](https://iiif.wellcomecollection.org/image/b21713613_0070.jp2/full/800%2C/0/default.jpg)