A dictionary of chemical solubilities : inorganic / by Arthur Messinger Comey.
- Comey Arthur Messinger, 1861-
- Date:
- 1896
Licence: Public Domain Mark
Credit: A dictionary of chemical solubilities : inorganic / by Arthur Messinger Comey. Source: Wellcome Collection.
Provider: This material has been provided by the Royal College of Physicians of Edinburgh. The original may be consulted at the Royal College of Physicians of Edinburgh.
75/544 (page 51)
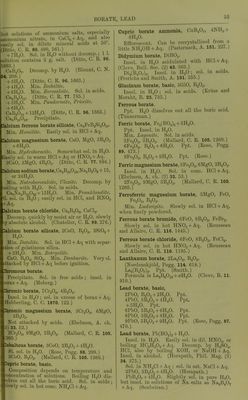
![Bismuthic acid, HBiO.,. Set- Bismuthic hydroxide. Potassium bismuthate, KBiOj. Sol. in H^O. (Arppe.) KH(HiO,).,. lusol. in H,,0. Not ilecouij). by boiling ii.,0. (Andro, C. R. L13. 860.) . No salts of HBiOs can exist. (Mnir and Carnegie, Cheni. Soc. 61. 77.) Bismuthyl bromide, BiOBr. Insol. in H.,0; sol. in moderately cone. IBr + Aq. BisOjBrg. Insol. in H2O ; easily sol. in onc^ HCl, or HNOj + Aq; less sol. in dil. INO, + Aq. BijjOisBry. As the preceding comp. (Muir.) liamuthyl chloride, BiOCl. Insol. in H.,0 or dil. acids. Sol. in cone. CI, or HNOa + Aq. + H„0. (Heintz, Pogg. 63. 55.) + 3H.,0. (PhiUips, Br. Arch. (1) 39. 41.) BirOsCls. (Arppe.) BiCCl.,. Insol. in H.,0 ; sol. in hot HCl, or NOa + Aq. (Muir.) ismuthyl fluoride, BiOF. Insol. in H2O ; sol. in HCl, HBr, or HI + Aq. ott and Muir, Cheni. Soc. 33. 139.) BiOF, 2HF. Insol. in HgO. smuthyl iodide, BiOI. Not deeonip. by H._,0 or alkaline solutions. •1. in HCl + Aq. Decorap. by HNOa + Aq. chneider, J. pr. 79. 424.) Insol. in KCl, or KI + Aq. Bila, 5Bi203. Ppt. SI. sol. in HCoH:,0„ + Not decomp. by HoO. (Fletcher and ibper, Pharni. J. (3) 13. 254.) 4Bii3, 5Bi.,0:,. Easily sol. in HCl + Aq. scomp. l)y HNOs + Aq. SI. attacked by 04; soniewliat sol. in H2C4H40q, and C4H40,, + Aq. lOl. in (NH4),S, and KOH + Aq. (Storer's ct.) authyl sulphide, BigO.,S. |[Herniann, .J. ]ir. 76. 452.) jijOjjS. Insol. in HjO. (Schecpenberg, [C. 1889, 2. 641.) in. Karelinite. :ic acid. ^ee Boric acid. pfee Tt^raborate, sodium. ic acid, anhydrous, B.p^. P«« Boron </ /oxide. rtoboric acid, HBO.^. ol. in HjO. Ortho\iox\c acid, H3BO3. Sol. in 33 ptH. HoO at 10°. 01 20°. „ 8 „ „ 100. (Berzelins.) Sol. in 20 iits. H2O at 18-75°. (Abl.) _ 100 pts. lUO at 100° dissolve 2 pts. (Ure s Uict.) 1 pt. crystallised acid dissolves in— 25-66 pts. H2O at 19°. 14-88 „ „ 25°. 12-66 „ „ 37-5 . 10-16 „ „ 50°. 6-12 „ „ 62-5°. 4-73 „ „ 75°. 3-55 ,, „ 87-5°. 2-97 „ „ 100°. Or, 100 pts. H,0 dissolve at— ir 3-9 pts. H,BO;, 25° 6'8 ,, ,, 37-5° 7'8 ,, ,, 50° 9-8 „ 62-5° 16-0 „ 75° 21-0 „ 87-5° 28-0 „ 100° 34-0 „ Or, sat.,aqueous solution contains at- 19° 3-75 % H3BO3. 25° 6-27 „ „ 37-.5° 7-32 „ „ 50° 8-96 „ „ 62-5° 14-04 „ „ 75° 17;44 „ „ 87-5° 21-95 ,, ,, 100° 25-17 „ „ (Brandes and Firnhaber, Arch. Pharm. 7. 50.) 1 liti-e HgO dissolves at- 0° 19-47 g- H3BO, 12° 29-20 i > ) J 20° 39-92 ) > >) 40° 69-91 ) > )) 62° 114-16 >» > ? 80° 168-15 ) f J) 102° 291-16 J) J > (Ditte, C. R. 86. 1069.) Sp. gr. of H3BO3 +Aq at 15°. % H3BO:, Sp. gr. % H3BO3 Sp. gr. 1 1 -0034 4 1-0147 2 1-0069 Sat. sol. 1-015 3 1-0106 (Gerlach, Z. anal. 28. 473.) Sp. gr. of HaBOs+Aq sat. at 8°=1-014. (Anthon, A. 24. 241.) S)). gr. of HsBOs+Aq sat. at 15°=l-0248. (Stolba. J. pr. 90. 457.) Volatile with steam. More sol. in dil. IICl + A(i than in IT„0. Sol. in warm cone. H.,S04, HCl, or UNO., + Ati. Sol. in 6 pts. alculiol (Wittstein), 5 i)t.s. boil- ing alcoliol (Wcnzcl). Only traces di.ssolvc in anhydnms etiier. (Schilf.) Sol. in 100 pts. ether. (Hager's Comm.) Sol. in .several essen- tial oils.](https://iiif.wellcomecollection.org/image/b21713613_0075.jp2/full/800%2C/0/default.jpg)