A dictionary of chemical solubilities : inorganic / by Arthur Messinger Comey.
- Comey Arthur Messinger, 1861-
- Date:
- 1896
Licence: Public Domain Mark
Credit: A dictionary of chemical solubilities : inorganic / by Arthur Messinger Comey. Source: Wellcome Collection.
Provider: This material has been provided by the Royal College of Physicians of Edinburgh. The original may be consulted at the Royal College of Physicians of Edinburgh.
83/544 (page 59)
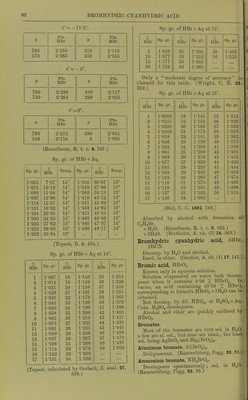
![BROMHYDRIC ACID V. Bousdorll'.) Cerium bromaurate, CeAuBre+SH^O. Sol. iiv Up. (I-'liu, Hull. S..C. (-2) 21. 533.) Didymium bromaurate, DiAuHr8 +9H.p. \ , i-y ilfli.iuosceut. Sol. in H..0. (Clevc.) l>aiithanum bromaiirate, LaAiiBr6 +9H.p. Sol. in H.O. (CU've.) Magnesium bromaurate. Di'liiiui'.scont in moist air. Manganese bromaurate. D.'li.liu'scent. (v. Bonsdorll.) Potassium bromaurate, KAulii-j. SI. sol. in H,p. iMore sol. in cold alcohol than in H„0. (v. Bons.loril'.) + 2H.,0. Sol. in 5-12 pts. H.,0 at 15°, 1-56 pts. at 40% and 0-48 pt. at 67°. Decomp. by tlu-r. SI. sol. in KBr + A(i. (Schottltinder, 217. 314.) + 5H.,0. Efflorescent, (v. Bonsdorff.) Rubidium bromaurate, RbAuBr4. As ca'siuiii bromaurate. Samarium bromaurate, SniAuBr,; +IOH.2O. \'ery deliquescent. (Cleve, Bull. Soc. (2) 43. 165.) Sodium bromaurate, NaAuBrj. Slowly sol. in HjO. (v. BonsdortF.) Zinc bromaurate, Zn(AnBr4)2. Very dt'liqiu'scent. (v. Bonsdorff.) Bromauricyanhydric acid. Not known in free state. Barium bromauricyanide, Ba[An(CN)2Br2]2 + 10H,,O. Very sol. in iiot or cold HgO, also in alcohol. (Lindl)om, Lund. Univ. Arsk. 12. No. 6.) Cadmium bromauricyanide,Cd[Au(CN)2Br2]..+ 6H.,0. Very sol. in hot or cold H2O, but solution is unstable. (Lindbom.) Calcitim bromauricyanide, Ca[Au(CN)2Br2]3 + lOH.p. Extremely sol. in H2O and alcohol. (Lind- lom.) Co[Au(CN)2Br2]2 + than Cobalt bromauricyanide, 9H2O Moderately sol. in HjO. Less sol. )ther broniauricyanides. (Lindbom.) PotasBitun bromauricyanide, KAu(CN)2Br2 Sol. in IL2O and alcohol. Boditun bromauricyanide, NaAu(CN)2Br2 + 2ll.p. Very sol. in H2O or alcohol. Strontium bromauricyanide, Sr[Au(CN)2Br2]2 + xlLf). Very sol. in HjO or alcohol. Zinc ^bromauricyanide, Zn[Au(CN)2Br2]2 + Easily sol. in cold or hot HgO. Bromhydric acid, HBr. Very sol. in H.jO. The most concentrated HBr + Aq has a sp. gr. of 1 -78, and contains 82-02 % H Br. (Champion and Pellat, C. R. 70. 620.) This, or a weak acid on heating leaves a residue, which dis- tils unchanged at 125-125-5 under 758 mm. pressure, a nd contains 48-17 % HBr (Top.soe) ; at 126' under 758 mm. prcssuro, and contains 46-83 % HBr (Bineau) ; and has sp. gr. = 1-486 at 20 (Bineau) ; sp. gr. = 1 -48 at 20° (Champion and Pellat) ; sp. gr. =1-49 at 20 (Topsoii). According to Roscoe (A. 116. 214) an acid of constant composition, obtained by boiling a stronger or a weaker acid, if distilled^under 752-762 mm. pressure, contains 47-38-47*86 % HBr, and boils at 126° at 760 mm. pressure ; but the composition is dependent on the pressure, as, for example, under 1952 mm. pressure, the residue boils at 153°, and contains 46-3 % HBr. (Roscoe.) By conducting dry air through HBr-l-Aq an acid is obtained containing 51-65 % HBr if at 16°, and 49-35 % HBr if at 100° (Roscoe.) 1 vol. HoO dissolves 600±vols. HBr at 10°. (Berthelot, C. R. 76. 679.) 1 pt. H2O at t° and 760 mm. pressure dissolves pts. HBr. t° Pts. : HBr t Pts. HBr t Pts. HBr -25 2-550 -5 2-280 + 50 1-715 -20 2-473 0 2-212 + 75 1-505 -15 2-390 -HO 2-103 + 100 1-300 -10 2-335 + 25 1-930 (Roozeboom, R. t. c. 4. 107.) Absorption by 1 pt. H2O at t° and p pressure in mm. t°= -25°. Pts. Pts. 1' HBr P HBr 760 2-550 100 2-056 300 2-263 1 1-755 140 2-120 0-5 1-10 t°= - 20°. Pts. Pts. P HBr P HBr 760 2-473 130 2-056 375 2-267 20 1-850 180 2-119 t°= - •15°. P Pts. Pts. HBr P HBr 760 2-390 175 2-0.^6 470 2-266 102 1-980 250 2-119](https://iiif.wellcomecollection.org/image/b21713613_0083.jp2/full/800%2C/0/default.jpg)