A dictionary of chemical solubilities : inorganic / by Arthur Messinger Comey.
- Comey Arthur Messinger, 1861-
- Date:
- 1896
Licence: Public Domain Mark
Credit: A dictionary of chemical solubilities : inorganic / by Arthur Messinger Comey. Source: Wellcome Collection.
Provider: This material has been provided by the Royal College of Physicians of Edinburgh. The original may be consulted at the Royal College of Physicians of Edinburgh.
88/544 (page 64)
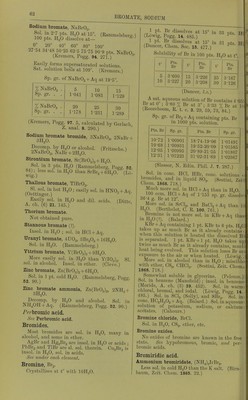
![BROMOPHOSPHATOPLATINAMINE PHOSPHATE BromophosphatoplatincZiamine phos- phate, BiPt(N2H6)2 + 2H.,0. \ / PO, SI. sol. in HoO. (Cleve.) Bromoplatinamine bromide, Bi2Pt(NH,Br)2. SI. sol. iu H2O. (Cleve, Sv. V. A. H. 10, 9. 31.) nitrite, Br2Pt(NH3N02)2. Very si. sol. in HoO. (Cleve.) BromoplatinfZzamine bromide, Br.,Pt(N2H6)2Br2. Only si. sol. in hot HgO. (Cleve.) chloride, Br2Pt(N2H6)2Cl2. Very si. sol. in HgO. (Cleve.) fZichromate, Br2Pt(N2H6)2Cr207. SI. sol. in H2O. nitrate, Br2Pt(]Sr2H8N03)2. SI. sol. in cold, rather easily sol. in hot n„0. (Cleve.) phosphate, Br2Pt[N2HBPOo(OH).2]2 + 2H2O. Rather easily .sol. in hot HoO. (Cleve.) sulphate, BroPt(NoHg).^04. Very si. sol. in H2O. Bromoplatin»io7iofZiamine nitrate, Br pt(NH.)2N0:, ^^^^^ NH3NO3 +^2^. Easily sol. in H2O. sulphate, Br2Pt(^y«)2so, + H2O. Moderately sol. in H.,0. (Cleve.) Bromoplatin.s<'»w'cZ/amine bromide, Br3Pt(NH3)2Br. SI. sol. in cold H2O. (Cleve.) Bromorfiplatinc??amiiie anhydronitrate, (NH3NH2)2 Sol. in HNOg + Aq. chloride, Br2Pt2(N2Ho)4Cl4. Ppt. (Cleve.) nitrate, Br2Pt2(N2Ho)4(N03)4 + 2H2O. Moderately sol. in hot HgO. sulphate, Br2Pt^(N2H6)4(S0,)2 + 2H2O. Ppt. (Cleve.) Bromoplatinic acid, HgPtBrg + 9H2O. Very deliquescent, and sol. in HoO, alcohol, ether, chloroform, or acetic acid. (Topsoe, J. B. 1868. 273.) Ammoniiun bromoplatinate, (NH4)oPtBr8. Sol. in 200 pts. HoO at If) (Topsoe.) 100 pts. (NH4)2PtBr„ +A(i sat. at 20° contain 0-.59 pt. dry salt. (Halberstadt, B. 17. 2965.) Barium bromoplatinate, BaPtBr8 +lOlIoO. SI. deliquescent. Very sol. in HgO. Calcium bromoplatinate, CaPtBr6 +12H0O. SI. deliquescent. Very sol. in HoO. Cobalt bromoplatinate, CoPtBrg + 12H20. Deliquescent. Copper bromoplatinate, CuPtBrg + 8H2O. Very deliquescent; sol. in HjO. Lead bromoplatinate, PbPtBrg. Easily sol. in HoO, but deconip. by large amount. Magnesium bromoplatinate, MgPtBrg + I2H2O. Not deliquescent. Manganese bromoplatinate, MnPtBrg + 6H20. Sol. in H2O. + 12H2O. Sol. inH20. Nickel bromoplatinate, NiPtBrB + 12H20. Deliquescent. Potassium bromoplatinate, KoPtBrg. SI. sol. in HoO. Insol. in alcohol, (v. Bonsdorff, Pogg.l9. 344.) Sol. in 10 pts. boiling (Pitkin, C. N. 41. 218.) 100 pts. KoPtBrg + Aq sat. at 20° contain 2-02 pts. dry salt. (Halberstadt, B. 17. 2962.) Sodium bromoplatinate, Na2PtBrg + 6H0O. Easily sol. in H„0 and alcohol. Strontium bromoplatinate, SrPtBrg +lOHoO. SI. deliquescent. Very sol. in H2O. Zinc bromoplatinate, ZnPtBru +12H.)0. Sol. in H2O. Bromoplatinocyanhydric acid, H.,Pt(CK)4Bro. Sec Perbromoplatinocyanhydric acid. Potassium bromoplatinocyanide, 5K,Pt(CN)4, KoPt(CN)4Br2+18HoO. Sol. in H2O. Bromopurpureochromium bromide, BrCr(KH3)BBro. Less sol. in HoO than chloropurpureo- chromium chloride. (Jorgensen, J. pr. (2) 25. 83.) bromoplatinate, BrCr(NH3)3 PtBrg. (Jorgensen, I.e.) chloride, BrCr(NH3)6Cl2. More sol. in H2O than the bromide. (Jorgensen, I.e.) chromate, BrCr(]SrH3)5Cr04. Preciiiitate. (Jorgensen, I.e.) nitrate, BrCr(NH3)5(N03)2. More sol. than bromide and less than- chloride. (Jorgensen, I.e.) Bromopurpureocobaltic bromide, CoBr(NH3)5Br„. Sol. in 530 pts. H2O at 16°. Insol. im alcohol, NH4Br, KBr, or HBr + Aq. More sol. in hot HoO containing a little HBr. (Jorgen- sen, ,1. i.r. (2) 19. 49.)](https://iiif.wellcomecollection.org/image/b21713613_0088.jp2/full/800%2C/0/default.jpg)